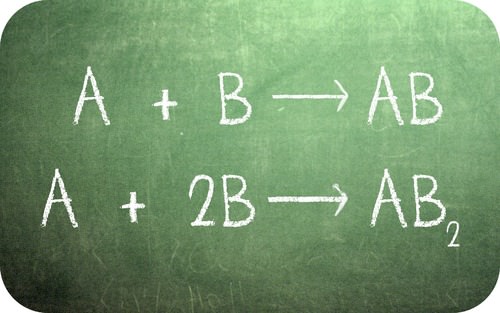质量比率计算
Section outline
-
What are the similarities and differences between these two equations?
::这两个方程式之间的相似和不同是什么?One of the fundamental laws of chemistry deals with the fact that we cannot (using chemical means) create or destroy matter. When a reaction is run, the number of atoms of each specific type must be the same on both sides of the equation. For some materials, it turns out that one can combine with a second element in more than one ratio. Carrying out mass ratio calculations helped establish the .
::化学的基本规律之一涉及这样一个事实,即我们不能(使用化学手段)创造或摧毁物质。当发生反应时,每种特定类型的原子的数量在等式的两侧必须相同。对于某些材料来说,事实证明,可以将第二个元素结合到一个以上的比例中。进行质量比率计算有助于建立这个比例。Mass Ratio Calculations
::质量比率计算Copper reacts with chlorine to form two compounds. A consists of 4.08 g of copper for every 2.28 g of chlorine. Compound B consists of 7.53 g of copper for every 8.40 g of chlorine. What is the lowest whole number mass ratio of copper that combines with a given mass of chlorine?
::铜与氯发生反应后形成两种化合物:A 由每2.28克氯4.08克铜组成;B 化合物由每8.40克氯7.53克铜组成。Step 1: List the known quantities and plan the problem.
::第1步:列出已知数量并规划问题。Known
::已知已知Compound A = 4.08 g Cu and 2.28 g Cl
::化合物A = 4.08克铜和 2.28克氯Compound B = 7.53 g Cu and 8.40 g Cl
::化合物B=7.53克铜和8.40克氯Apply the law of multiple proportions to the two compounds. For each compound, find the grams of copper that combine with 1.00 g of chlorine by dividing the mass of copper by the mass of chlorine. Then, find the ratio of the masses of copper in the two compounds by dividing the larger value by the smaller value.
::对两种化合物适用多重比例法则。 对于每一种化合物,通过将铜质量除以氯质量,找到与1.00克氯结合的铜克。然后,通过将较大值除以较小值,找到两种化合物中铜质量的比例。Step 2: Calculate.
::第2步:计算。Compound A
::A4.08克 Cu2.28克 Cl=1.79克 cu1.00克 ClCompound B
::化合物B 7.53 g Cu8.40 g Cl=0.896 g Cu1.00 g ClCompare the masses of copper per gram of chlorine in the two samples.
::比较两个样本中每克氯中每克铜的重量。
::1.79 g Cu(在化合物A)0.896 g Cu(在化合物B)=2.001=2:1The mass ratio of copper per gram of chlorine in the two compounds is 2:1.
::在两种化合物中,铜每克氯的质量比率为2:1。Step 3: Think about your result.
::步骤3:想想你的结果。The ratio is a small whole-number ratio. For a given mass of chlorine, compound A contains twice the mass of copper as does compound B.
::该比率是一个小的整数比率,对于某一量的氯,化合物A的铜含量是化合物B的两倍。Copper (II) Chloride (CuCl2) forms pale blue crystals Summary
::摘要-
The mass ratio gives the mass of an element that is found in combination with another element.
::质量比率给出一个元素的质量,该元素与另一个元素结合发现。
Review
::回顾-
What does the mass ratio between two elements tell us?
::两个元素之间的质量比例告诉我们什么? -
If we compare the mass ratio of elements in one compound to that in an second compound what can we learn? Give an example from the lesson above.
::如果我们比较一个化合物中元素的质量比和第二个化合物中元素的质量比,我们可以学到什么?从上面的教训中举一个例子。 -
In compound A, there is 6.3 g of hydrogen and 18.7 g of carbon, while in compound B there is 6.9 g of hydrogen and 41.0 g of carbon, what is the carbon to hydrogen mass ratio in each compound and how do these ratios compare?
::在化合物A中,氢为6.3克,碳为18.7克,而在化合物B中,氢为6.9克,碳为41.0克,每种化合物的碳与氢质量比率是多少? 这些比率如何比较? -
What are lowest ratios of hydrogen and carbon in compounds A and B? Predict their formulas
::化合物A和化合物B中氢和碳的最小比例是多少? 预测其公式
-
The mass ratio gives the mass of an element that is found in combination with another element.