百人规则和轨道填充图
章节大纲
-
Have you ever wondered what those load limit signs mean on a bridge?
::你有没有想过 桥上那些负载极限标志意味着什么?The sign above says that nothing over five tons is allowed because it will do damage to the structure. There are limits to the amount of weight that a bridge can support, there are limits to the number of people that can safely occupy a room, and there are limits to what can go into an .
::上面的标志表示,允许超过5吨的量都超过5吨,因为这会对建筑造成破坏。 一座桥梁能够支撑的重量有限度,能够安全占据一个房间的人数有限度,进入一个房间的人数也有限度。Hund’s Rule
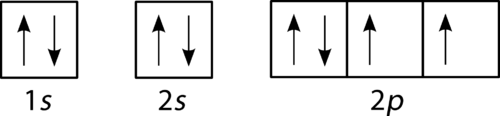
::百人规则The last of the three rules for constructing electron arrangements requires electrons to be placed one at a time in a set of orbitals within the same sublevel. This minimizes the natural repulsive forces that one electron has for another. Hund’s rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of the single electrons must have the same spin. The Figure shows how a set of three p orbitals is filled with one, two, three, and four electrons.
::构建电子安排的三项规则中,最后一项规则要求将电子一次放置在同一子层的一组轨道上。 这使得一个电子对另一个电子的自然厌恶力最小化。 一百条规则规定,在任何轨道被第二电子占用之前,每个等能量的轨道都由一个电子占据,每个单电子必须有一个相同的旋转。 图显示一组三颗轨道是如何装满一、二、三和四颗电子的。The 2p sublevel, for the elements boron (Z = 5), carbon (Z = 6), nitrogen (Z = 7), and oxygen (Z = 8). According to Hund’s rule, as electrons are added to a set of orbitals of equal energy, one electron enters each orbital before any orbital receives a second electron.
::2p 子级,对于元素boron(Z = 5)、碳(Z = 6)、氮(Z = 7)和氧(Z = 8),2p 子级,根据洪德规则,由于电子被添加到一套同等能量的轨道上,在任何轨道获得第二电子之前,每进入一个轨道。Orbital Filling Diagrams
::轨道填充图An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular . In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal and sublevel. Electrons are indicated by arrows inside the circles. An arrow pointing upwards indicates one spin direction, while a downward pointing arrow indicates the other direction. The orbital filling diagrams for hydrogen, helium, and lithium are shown in Figure .
::轨道填充图是代表特定区域中所有电子安排的更直观的方法。在轨道填充图中,单个轨道以圆形(或方形)显示,一个子层内的轨道以水平顺序排列。每个子层由主和子层标注。电子以圆内箭头标出。向上指向一个旋转方向的箭头,向下指向另一个方向的箭头则指向另一个方向。氢、氦和锂的轨道填充图在图中显示。Orbital filling diagrams for hydrogen, helium, and lithium.
::氢、和锂的轨道填充图。According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium. The next is lithium and necessitates the use of the next available sublevel, the 2 s .
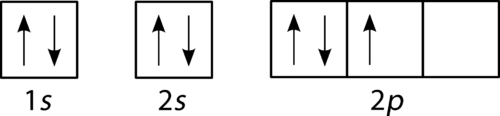
::根据Aufbau工艺,子级和轨道都装满了电子,以便增加能量。由于子级仅包括一个轨道,因此第二电子仅与第一电子对齐,如氦。下一个是锂,需要使用下一个可用的子级,即第二电子。The filling diagram for carbon is shown in Figure . There are two 2 p electrons for carbon and each occupies its own 2 p orbital.
::碳的填充图见图 。碳的填充图为 2,2p 电子,各占2p 轨道。Orbital filling diagram for carbon.
::碳轨道填充图。Oxygen has four 2 p electrons. After each 2 p orbital has one electron in it, the fourth electron can be placed in the first 2 p orbital with a spin opposite that of the other electron in that orbital.
::氧有4个 2p 电子。每2p 轨道内有一电子后,第四电子可以放置在第一个 2p 轨道内,其旋转与该轨道内另一电子的旋转相对。Orbital filling diagram for oxygen.
::氧气轨道填充图。Summary
::摘要-
Hund’s rule specifies the order of electron filling within a set of orbitals.
::一百人的规则具体规定了一组轨道内电子填充的顺序。 -
Orbital filling diagrams are a way of indicating electron locations in orbitals.
::轨道填充图是表明轨道中电子位置的一种方法。
Review
::回顾-
State Hund’s rule.
::国家洪德规则。 -
What is an orbital filling diagram?
::什么是轨道填充图? -
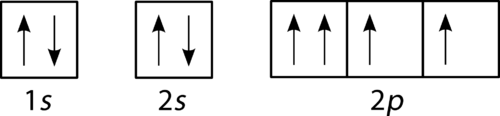
Is the diagram in
Figure
correct? Explain your answer.
::图中的图表是否正确? -
Is the diagram in
Figure
correct? Explain your answer.
::图中的图表是否正确?
-
Hund’s rule specifies the order of electron filling within a set of orbitals.