Anion 形成
Section outline
-
A pressed glass open salt dish made in the early 1830s.
::1830年代初制制成的玻璃开胃盐盘。How do you make chlorine safe to eat?
::如何使氯安全进食?How do you transform a deadly into something you can sprinkle on your eggs and eat for breakfast? Chlorine in its free form is very dangerous if you breathe the fumes or come in contact with the gas. However, after reaction with sodium, we have sodium chloride formed as the sodium gives up an to chlorine which accepts the electron to form the chloride .
::如何把致命的氯化成可以洒在鸡蛋上和吃早餐的东西?自由形式的氯如果吸入烟雾或接触气体,是非常危险的。然而,在与钠发生反应后,当钠向接受电子形成氯的氯中放弃氯时,我们形成了氯化钠。Anions
::阴离Anions are the negative formed from the gain of one or more electrons. When atoms gain electrons, they often do so until their outermost principal energy level achieves an octet. This process is illustrated below for the fluorine, oxygen, and nitrogen.
::当原子获得电子时,当原子获得电子时,它们往往会这样做,直到它们最外围的主要能量水平达到八点。 这一过程在下文中以氟、氧和氮为例。
::F+eF-1s22s22s22s22p6(octe)O+2e-O2-1s22s22s22p41s22s22p6(octe)N+3e-N3-1s22s22s22s22s22p6(octe)All of these anions are isoelectronic with each other and with neon. They are also isoelectronic with the three from the previous section. Under typical conditions, three electrons is the maximum that will be gained in the formation of anions.
::所有这些离子都是相互之间电子化的,并且有射线,与上一节的3个是电子化的。在典型条件下,三种电子是形成离子时获得的最大值。Outer are constant within a group, so this pattern of ion formation repeats itself for Periods 3, 4, and following (see Figure ).
::外体在一个组内是不变的,因此这种离子形成模式在第三、第四和以后的期间会重复(见图)。Ion charges.
::收费。It is important not to misinterpret the concept of being isoelectronic. A sodium ion is very different from a neon atom because the nuclei of the two contain different numbers of . One is an essential ion that is a part of table salt , while the other is an unreactive gas that is a very small part of the atmosphere. Likewise, sodium ions are very different than magnesium ions, fluoride ions, and all the other members of this isoelectronic series (N 3− , O 2− , F − , Ne, Na + , Mg 2+ , Al 3+ ).
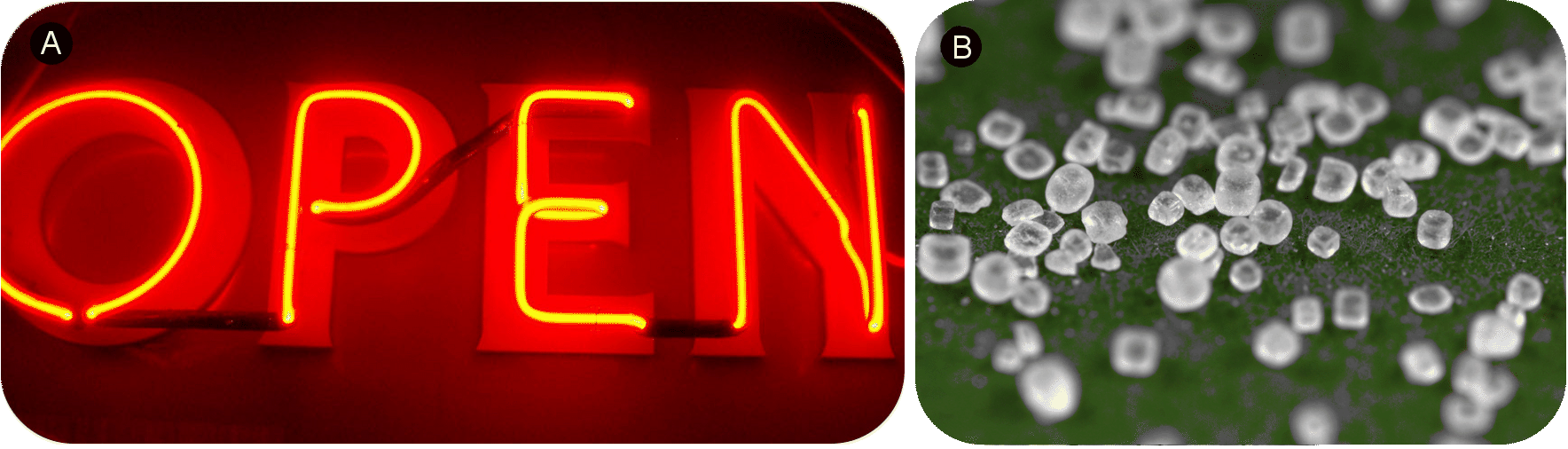
::重要的是不要曲解电子化的概念。 钠离子与纳米原子非常不同, 因为两者的核包含不同的数字。 一种是表盐的一部分基本离子, 而另一种是大气中非常小的一部分非反应性气体。 同样, 钠离子与镁离子、氟离子和本等电子系列的所有其他成员( N3-, O2-, F--Ne, Na+, Mg2+, Al3+)非常不同。Neon gas (A) and sodium chloride crystals (B). Neon atoms and sodium ions are isoelectronic. Neon is a colorless and unreactive gas that glows a distinctive red-orange color in a gas discharge tube. Sodium ions are most commonly found in crystals of sodium chloride, ordinary table salt.
::神经气体(A)和氯化钠晶体(B)是异电子的。纳米原子和钠离子是异电子的。神经是一种无色和无反应的气体,在气体排放管中发光一种独特的红色。在氯化钠和普通桌盐的晶体中最常见的是钠离子。Summary
::摘要-
Anions are negative ions formed by accepting electrons.
::蚂蚁是接受电子形成的负离子。 -
The outermost principal energy level usually is an octet.
::最外边的主要能源水平通常为八点。
Review
::回顾-
What is an anion?
::什么是阴离子? -
Write the electronic configurations for the chlorine atom and the chloride anion.
::写下氯原子和氯化阴离子的电子配置。 -
What does isoelectronic mean?
::电子电子是什么意思?
-
Anions are negative ions formed by accepting electrons.