电离化合物的物理属性
章节大纲
-
In nature, the ordered arrangement of ionic solids gives rise to beautiful crystals. (A) Amethyst – a form of quartz, SiO 2 , whose purple color comes from iron ions. (B) Cinnabar – the primary ore of mercury is mercury(II) sulfide, HgS. (C) Azurite – a copper mineral, Cu 3 (CO 3 ) 2 (OH) 2 . D) Vanadinite – the primary ore of vanadium, Pb 5 (VO 4 ) 3 Cl.
::在自然界中,定购的离子固体安排会产生美丽的晶体。 (A) Amethyst — 一种石英形式,SiO2,其紫色来自铁离子。 (B) Cinnabar — 汞的主要矿石是汞(II)硫化物,汞(C) Azurite — 铜矿,Cu3(CO3)(OH)2. D — 范安地锡的主要矿石,Pb5(VO4)3Cl。What produces colored crystals?
::什么产生彩色晶体?The figure above shows just a few examples of the color and brilliance of naturally occurring ionic crystals. The regular and orderly arrangement of in the crystal lattice is responsible for the various shapes of these crystals, while transition metal ions give rise to the colors.
::上图仅举几个例子说明自然产生的离子晶体的颜色和亮度。晶体晶体的常规和有序安排是这些晶体不同形状的原因,而过渡性金属离子产生颜色的原因。Physical Properties of Ionic Compounds
::电离化合物的物理属性Melting Points
::熔点Because of the many simultaneous attractions between and that occur, ionic crystal lattices are very strong. The process of an requires the addition of large amounts of energy in order to break all of the in the crystal. For example, sodium chloride has a melting point of about 800°C.
::由于同时发生许多引力,因此离子晶体层非常坚固。一个过程需要增加大量能量才能打破晶体中的所有能量。例如,氯化钠的熔点约为800°C。Shattering
::摇摇Ionic compounds are generally hard, but brittle. Why? It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to shift relative to its neighbor. However, when that happens, it brings ions of the same charge next to each other (see Figure ). The repulsive forces between like-charged ions cause the crystal to shatter. When an ionic crystal breaks, it tends to do so along smooth planes because of the regular arrangement of the ions.
::离子化合物一般是硬的,但易碎的。 为什么? 它需要大量的机械力量, 比如用锤子击打晶体, 才能迫使一层离子相对于其邻居移动。 但是, 当发生这种情况时, 离子会相互产生相同的电荷( 见图 ) 。 类似电离子之间的可憎力量导致晶体碎裂。 当离子晶体断裂时, 它会随着离子的正常排列而滑动。(A) The sodium chloride crystal is shown in two dimensions. (B) When struck by a hammer, the negatively-charged chloride ions are forced near each other and the repulsive force causes the crystal to shatter.
:A) 氯化钠晶体以两个维度显示。 (B) 被锤子击打时,负压氯化物离子被相互逼近,令人厌恶的力量导致晶体碎裂。
Conductivity
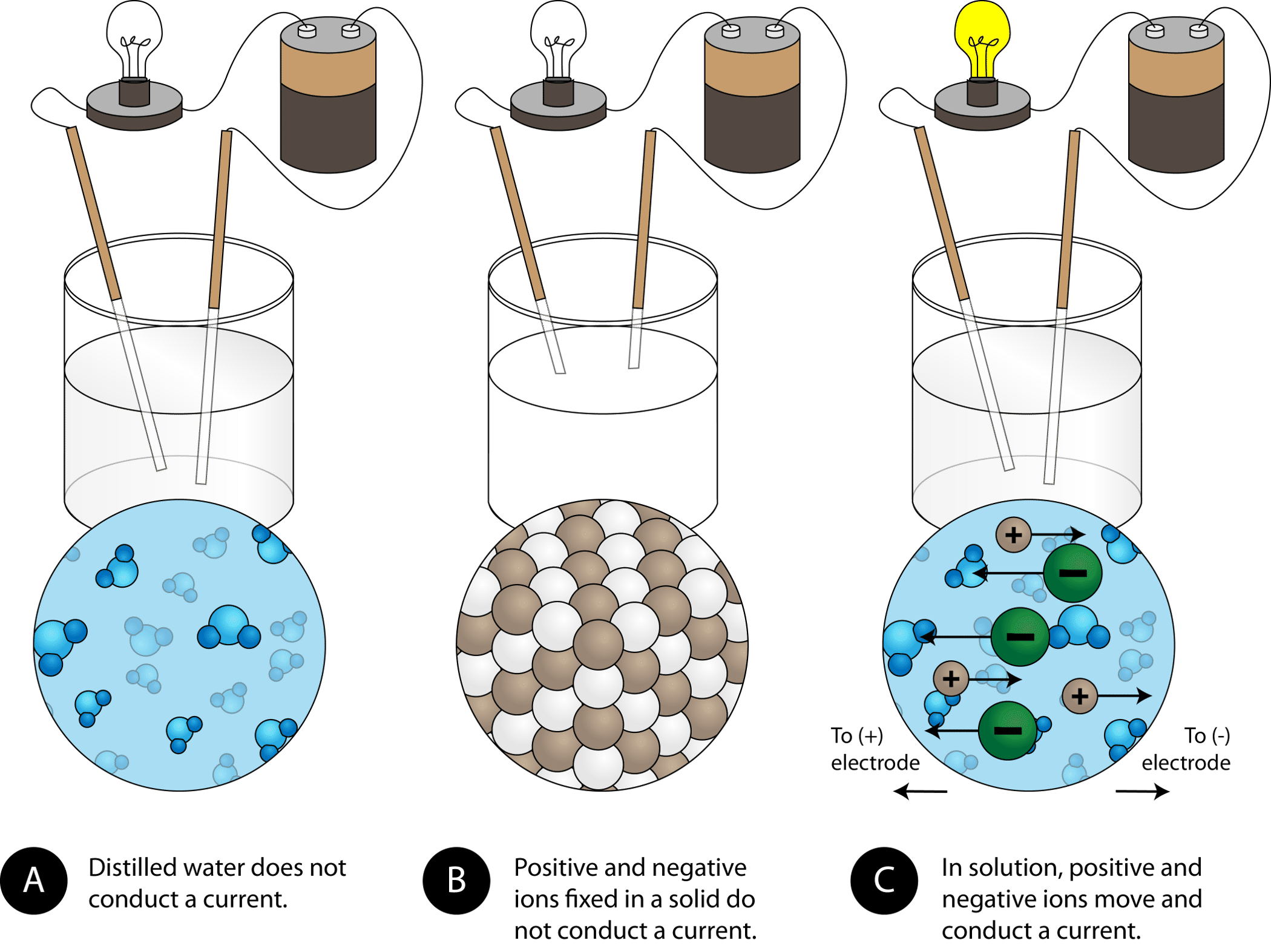
::传导能力Another characteristic property of ionic compounds is their electrical conductivity. The figure below shows three experiments in which two electrodes that are connected to a light bulb are placed in beakers containing three different substances .
::离子化合物的另一个特征特性是其导电性。下图显示了三个实验,其中两个与灯泡相连的电极放在含有三种不同物质的油箱中。(A) Distilled water does not conduct electricity. (B) A solid ionic compound also does not conduct. (C) A water solution of an ionic compound conducts electricity well.
:A) 蒸馏水不能用电。 (B) 固态离子化合物也不能用电。 (C) 离子化合物的水溶液电力很好。
In the first beaker, distilled water does not conduct a current because water is a molecular . In the second beaker, solid sodium chloride also does not conduct a current. Despite being ionic and thus composed of charges particles, the solid crystal lattice does not allow the ions to move between the electrodes. Mobile charged particles are required for the circuit to be complete and the light bulb to light up. In the third beaker, the NaCl has been dissolved into the distilled water. Now the crystal lattice has been broken apart and the individual positive and negative ions can move. Cations move to one electrode , while anions move to the other, allowing electricity to flow (see Figure ). Melting an ionic compound also frees the ions to conduct a current. Ionic compounds conduct an electric current when melted or dissolved in water.
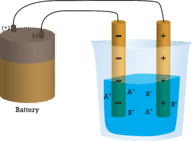
::在第一个瓶子中,蒸馏水不会流水,因为水是分子。在第二个瓶子中,固体氯化钠也不会流水。尽管是离子的,因此由电荷颗粒组成,但固体晶晶体板不允许离子在电极之间移动。要完成电路和灯泡照明,需要移动充电颗粒。在第三个瓶子中,NaCl已经溶入蒸馏水中。现在晶体板已经破裂,个人正反离子可以移动。电离子移到一个电极,而电离子移到另一个,允许电流(见图 )。熔融一个离子化合物也释放离子进行电流。在水中熔化或溶解时,ICl化合物会形成电流。In an ionic solution, the A + ions migrate toward the negative electrode, while the B − ions migrate toward the positive electrode.
::在离子溶液中,A+离子移向负电极,而B-离子移向正电极。Summary
::摘要-
Ionic compounds have high melting points.
::电离化合物熔点高。 -
Ionic compounds are hard and brittle.
::电离化合物是硬的和易碎的。 -
Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not.
::离子化合物和熔化的离子化合物的溶液进行电动,但固体材料不进行电动。
Review
::回顾-
Why are ionic compounds brittle?
::为什么离子化合物易碎? -
Why are melting points high for ionic compounds?
::为什么离子化合物的熔点高? -
What happens when an electric current is passed through a solution of an ionic compound?
::当电流通过离子化合物溶液时会怎样?
Explore More
::探索更多Watch the video below and answer the following questions:
::看下面的录像,回答下列问题:-
Do all ionic compounds form crystals?
::所有离子化合物都形成晶体吗? -
Will melted ionic compounds conduct electricity?
::融化的离子化合物能供电吗? -
What are the melting and boiling points of KI?
::KI的熔点和沸点是什么?
-
Ionic compounds have high melting points.