影响气体压力的因素
Section outline
-
How high does a basketball bounce?
::篮球弹跳有多高?The pressure of the air in a basketball has to be adjusted so that the ball bounces to the correct height. Before a game, the officials check the ball by dropping it from shoulder height and seeing how far back up it bounces. What would the official do if the ball did not bounce up as far as it is supposed to? What would he do if it bounced too high?
::篮球中的空气压力必须调整,以使球弹到正确的高度。 在比赛前,官员们检查球的高度,从肩部高度下降,看球的反弹有多远。 如果球没有按预想的那样反弹,官员会怎么做?如果球弹得太高,他会怎么做?The pressure inside a container is dependent on the amount of inside the container. If the basketball does not bounce high enough, the official could remedy the situation by using a hand pump and adding more air to the ball. Conversely, if it bounces too high, he could let some air out of the ball.
::容器内的压力取决于容器内的数量,如果篮球弹得不够高,官员可以通过使用手泵和在球中增加更多的空气来补救这种情况,相反,如果弹得太高,他可以让一些空气从球中出来。Factors Affecting Gas Pressure
::影响气体压力的因素Recall from the kinetic-molecular theory that gas particles move randomly and in straight lines until they elastically collide with either other gas particles or with one of the walls of the container. It is these collisions with the walls of the container that defines the pressure of the gas. Four variables are used to describe the condition of a gas. They are pressure , volume , temperature , and the amount of the gas as measured by the number moles "> . We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample.
::从动能分子理论中回顾,气体粒子在直线上随机移动,直到它们与其他气体粒子或容器的墙壁发生剧烈碰撞,正是这些与容器墙壁的碰撞决定了气体的压力。用四个变量来描述气体的状态。它们是压力(P)、体积(V)、温度(T)以及用摩尔数测量的气体数量。我们将分别审查气体的体积、温度和数量如何影响封闭气体样品的压力。
Amount of Gas
::天然气数量Figure shows what happens when air is added to a rigid container . A rigid container is one that is incapable of expanding or contracting. A steel canister is an example of a rigid container.
::图中显示了在硬质容器中添加空气时发生的情况。硬质容器是无法扩展或订约的容器。钢罐是硬质容器的一个例子。Increase in pressure with increase in number of gas particles.
::随着气体粒子数量的增加,压力增加。The canister on the left contains a gas at a certain pressure. The attached air pump is then used to double the amount of gas in the canister. Since the canister cannot expand, the increased number of air molecules will strike the inside walls of the canister twice as frequently as they did before. The result is that the pressure inside the canister doubles. As you might imagine, if more and more air is continually added to a rigid container, it may eventually burst. Reducing the number of molecules in a rigid container has the opposite effect and the pressure decreases.
::左侧的罐体内含有一定压力的气体。 然后, 随附的气泵将用来将罐体内的气体量翻一番。 由于罐体不能扩张, 增加的空气分子数量将比以前多两倍地击中罐体内墙壁。 结果是罐体内的压力翻倍。 正如你可能想象的那样, 如果不断将越来越多的空气添加到硬质容器中, 它最终可能会爆裂。 减少硬质容器中的分子数量具有相反的效果, 压力也会降低 。Volume
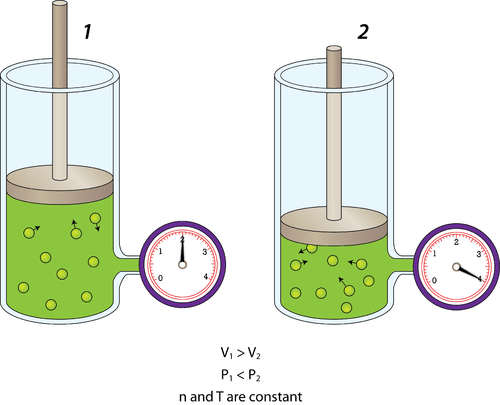
::量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量量Pressure is also affected by the volume of the container. If the volume of a container is decreased, the gas molecules have less space in which to move around. As a result, they will strike the walls of the container more often and the pressure increases.
::如果容器的容量减少,气体分子移动的空间就会减少,因此,气体分子会更频繁地撞击容器的墙壁,压力也会增加。Figure shows a cylinder of gas whose volume is controlled by an adjustable piston. On the left, the piston is pulled mostly out and the gauge reads a certain pressure. On the right, the piston has been pushed so that the volume of the enclosed portion of the container where the gas is located has been cut in half. The pressure of the gas doubles. Increasing the volume of the container would have the opposite effect and the pressure of the gas would decrease.
::图中显示一个气瓶的体积由可调整活塞控制。 在左侧,活塞大部分被拉出,测量仪读取一定的压力。 在右侧,活塞被推动,使气体所在容器的封闭部分的体积减半。气体的压强加倍。增加容器的体积会产生相反的效果,气体的压力也会减少。Decrease in gas volume produced increase in gas pressure.
::天然气量减少导致气体压力增加。Temperature
::温度It would be very unadvisable to place a can of soup over a campfire without venting the can. As the can heats up, it may explode. The kinetic-molecular theory explains why. The air inside the rigid can of soup is given more by the coming from the campfire. The kinetic energy causes the air molecules to move faster and they impact the container walls more frequently and with more force. The increase in pressure inside may eventually exceed the strength of the can and it will explode. An additional factor is that the soup may begin which will then aid even more gas and more pressure to the inside of the can.
::将一罐汤放在营火上而不喷出罐头将是非常不可取的。 当罐头能够加热时, 它可能会爆炸。 动能分子理论解释了原因。 硬汤罐中的空气会更多地从营火中流出。 动能会让空气分子更快移动, 更频繁地、 更强烈地撞击容器墙壁。 室内压力的增加可能最终超过罐子的强度, 并且会爆炸。 另外一个因素是, 汤可能会开始, 这样会帮助更多的气体, 给罐子内部带来更大的压力 。Shown in Figure is a cylinder of gas on the left that is at room temperature (300 K). On the right, the cylinder has been heated until the Kelvin temperature has doubled to 600 K. The kinetic energy of the gas molecules increases, so collisions with the walls of the container are now more forceful than they were before. As a result, the pressure of the gas doubles. Decreasing the temperature would have the opposite effect, and the pressure of an enclosed gas would decrease.
::图中显示的是左侧一个气瓶,温度(300K)在室温(300K)下。在右侧,气瓶被加热,直到克尔文温度翻番到600K。气体分子的动能增加,因此与容器墙壁的碰撞现在比以前更加强烈。结果,气体的压力加倍。降低温度会产生相反的效果,封闭气体的压力也会降低。Increase in temperature produces increase in pressure.
::温度升高会增加压力。Summary
::摘要-
An increase in the number of gas molecules in the same volume container increases pressure.
::同一容量容器中气体分子数量的增加增加了压力。 -
A decrease in container volume increases gas pressure.
::集装箱数量减少会增加气体压力。 -
An increase in temperature of a gas in a rigid container increases the pressure.
::硬质容器中气体温度的上升会增加压力。
Review
::回顾-
What defines the pressure of a gas?
::什么定义气体的压力? -
Why does an increase in the number of molecules increase the pressure?
::为什么分子数量的增加会增加压力? -
Why does an increase in temperature increase the pressure?
::为什么气温升高会增加压力?
Explore More
::探索更多Use the below resource to answer the questions that follow.
::使用以下资源回答以下问题。-
What causes pressure?
::是什么造成压力? -
What happens when you let gas out of the container?
::当你把气体放出容器时会怎么样? -
If you increase the temperature, what happens to the pressure?
::如果温度升高,压力会怎样? -
Why does the pressure drop when you increase the volume?
::当你增加体积时,为什么压力会下降?
-
An increase in the number of gas molecules in the same volume container increases pressure.