真实和理想气体
章节大纲
-
Location, Location, Location
::地点、地点、地点The behavior of a molecule depends a lot on its structure. We can have two compounds with the same number of atoms and yet they act very differently. Ethanol (C 2 H 5 OH) is a clear that has a boiling point of about 79°C. Dimethylether (CH 3 OCH 3 ) has the same number of carbons, hydrogens, and oxygens, but boils at a much lower temperature (-25°C). The difference lies in the amount of intermolecular interaction (strong H-bonds for ethanol, weak van der Waals force for the ether).
::分子的行为取决于分子的结构。 我们可以有两种化合物, 原子数量相同, 但是它们的行为却非常不同。 乙醇( C2H5OH) 明显地具有约79°C的沸点。 二甲基乙醚( CH3OCH3) 的碳、 氢和氧数量相同, 但以低得多的温度( 25°C ) 沸腾。 区别在于分子间相互作用的数量( 乙醇的强性H- 博德、 乙醇的弱性范德华力 ) 。Real and Ideal Gases
::真实和理想气体An ideal gas is one that follows the laws at all conditions of temperature and pressure . To do so, the gas would need to completely abide by the kinetic-molecular theory . The gas particles would need to occupy zero volume and they would need to exhibit no attractive forces what so ever toward each other. Since neither of those conditions can be true, there is no such thing as an ideal gas. A real gas is a gas that does not behave according to the assumptions of the kinetic-molecular theory. Fortunately, at the conditions of temperature and pressure that are normally encountered in a laboratory, real gases tend to behave very much like ideal gases.
::理想气体是一种在所有温度和压力条件下都遵循定律的理想气体。 要做到这一点,气体需要完全遵守动能分子理论。气体粒子需要保持零体积,它们不需要表现出任何相互对立的吸引力。由于这两个条件都不是真实的,因此不存在理想气体。真正的气体是一种不符合动能分子理论假设的气体。幸运的是,在实验室通常遇到的温度和压力条件下,实际气体往往与理想气体非常相似。Under what conditions then, do gases behave least ideally? When a gas is put under high pressure, its molecules are forced closer together as the empty space between the particles is diminished. A decrease in the empty space means that the assumption that the volume of the particles themselves is negligible is less valid. When a gas is cooled, the decrease in of the particles causes them to slow down. If the particles are moving at slower speeds, the attractive forces between them are more prominent. Another way to view it is that continued cooling the gas will eventually turn it into a liquid and a liquid is certainly not an ideal gas anymore (see liquid nitrogen in Figure ). In summary, a real gas deviates most from an ideal gas at low temperatures and high pressures. Gases are most ideal at high temperature and low pressure.
::当气体处于高压下时,分子会被迫更紧密地聚集在一起,因为粒子之间的空隙会缩小。空空空空间的减少意味着粒子的体积本身可忽略不计的假设不那么有效。当气体被冷却时,粒子的减少会使它们减速。如果粒子以较慢的速度移动,它们之间的吸引力就会更加突出。另一种观点是,继续冷却气体最终会将气体变成液体,而液体肯定不再是理想的气体(见图 ) 。 总之,在低温和高压下,真正的气体会大多偏离理想的气体。气体在高温和低压下是最理想的。Nitrogen gas that has been cooled to 77 K has turned to a liquid and must be stored in a vacuum insulated container to prevent it from rapidly vaporizing.
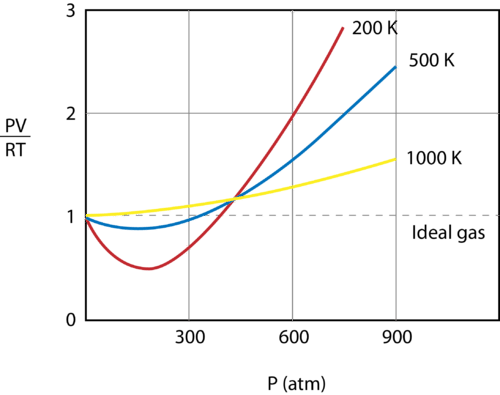
::被冷却到77K的氮气已变成液体,必须储存在真空隔热容器中,以防止其迅速蒸发。Figure shows a graph of plotted against pressure for 1 mol of a gas at three different temperatures - 200 K, 500 K, and 1000 K. An ideal gas would have a value of 1 for that ratio at all temperatures and pressures and the graph would simply be a horizontal line. As can be seen, deviations from an ideal gas occur. As the pressure begins to rise, the attractive forces cause the volume of the gas to be less than expected and the value of drops under 1. Continued pressure increase results in the volume of the particles to become significant and the value of rises to greater than 1. Notice, that the magnitude of the deviations from ideality is greatest for the gas at 200 K and least for the gas at 1000 K.
::图中显示一个PVRT图图,显示在三种不同温度 -- -- 200K、500K和1000K -- -- 一种气体的压力下,在三种不同的温度 -- -- 200K、500K和1000K -- -- 下,一个气压1兆瓦的压力下,一个理想气体的值为1,在所有温度和压力下,这个比率的值为1,而该图只是一条水平线。可以看到,与理想气体的偏差会发生。随着压力开始上升,吸引力使气体的体积低于预期,而光电RT的值下降到1之下。 持续压力增加的结果是粒子的体积变得巨大,而光电效应RT的值上升到超过1。注意,与理想性偏差的程度对于气体来说最大,为200K,对于气体来说最少为1000K。Real gases deviate from ideal gases at high pressures and at low temperatures.
::实际气体在高压和低温下偏离理想气体。The ideality of a gas also depends on the strength and type of intermolecular attractive forces that exist between the particles. Gases whose attractive forces are weak are more ideal than those with strong attractive forces. At the same temperature and pressure, neon is more ideal than water vapor because neon’s atoms are only attracted by weak dispersion forces, while water vapor’s molecules are attracted by relatively stronger . Helium is a more ideal gas than neon because its smaller number of electrons means that helium’s dispersion forces are even weaker than those of neon.
::气体的理想性也取决于颗粒之间存在的分子间诱人力量的强度和类型。 具有吸引力的气体比具有强大吸引力的气体更理想。 在同样的温度和压力下,纳米比水蒸气更理想,因为纳米原子只是被微弱的分散力量所吸引,而水蒸气分子则被相对强大的扩散力量所吸引。 比奈子更理想的气体,因为它的电量较少,这意味着的分散力量比的分散力量更弱。Summary
::摘要-
The properties of real gases and their deviations from ideality are described.
::报告叙述了实际气体的特性及其偏离理想的情况。
Review
::回顾-
What becomes more significant as the pressure increases?
::随着压力的增大,什么更加重要? -
Do the attractive forces between gas particles become more prominent at higher or lower temperatures?
::在高温或低温时,气体粒子之间的吸引力力量是否更加突出? -
Would HCl gas be more or less ideal than helium?
::氢化碳气体是否比更理想?
-
The properties of real gases and their deviations from ideality are described.