水的物理属性
Section outline
-
What is this pan used for?
::这个锅是用来做什么的?Water loss to the atmosphere is a significant problem in many parts of the world. When water supplies are low, anything that can be done to decrease water loss is important for farmers. An pan (seen above) can be used to measure how fast water evaporates in a given location. This information can be used as part of projects to develop ways to cut down on evaporation and increase the amount of usable water in a region.
::在世界许多地方,大气缺水是一个严重问题,当供水量低时,任何能够减少缺水量的措施对农民都很重要,一个锅(见上文)可用于测量特定地点水蒸发速度,这一信息可以作为项目的一部分,以制定减少蒸发和增加一个区域可用水量的方法。Properties of Water
::水的属性Compared to other of relatively low , ice melts at a very high temperature . A great deal of energy is required to break apart the hydrogen-bonded network of ice and return it to the state. Likewise, the boiling point of water is very high. Most molecular compounds of similar molar mass are at room temperature.
::与其他相对较低的冰层相比,冰在非常高的温度下融化。 打破含氢的冰层网络并将其归还给国家需要大量能量。 同样,水的沸点也非常高。 大多数类似摩尔质量的分子化合物都在室温下。Surface Tension
::表面紧张Water has a high surface tension (attraction between molecules at the surface of a liquid) because of its . Liquids that cannot do not exhibit nearly as much . Surface tension can be seen by the curved meniscus that forms when water is in a thin column such as a graduated cylinder or a buret.
::水因其表面高度张力(液体表面分子之间的吸引),其表面张力很高(液体表面的分子之间的吸引) 。水的液体不能显示出几乎同样多的液体 。 表面张力可以从在水的薄体(如分层的圆柱或薄膜)中形成时形成的弯曲的菜单中看出。The meniscus of water in a graduated cylinder forms because of water’s hydrogen bonding.
::因为水的氢气结合, 水的元云层以已毕业的气瓶形式形成。Vapor Pressure
::蒸气压力The vapor pressure of a liquid is the pressure of the vapor produced by evaporation of a liquid or solid above the liquid or solid in a closed container. The hydrogen bonding between liquid water molecules explains why water has an unusually low . Relatively few molecules of water are capable of escaping the surface of the liquid and enter the vapor phase . Evaporation is slow and thus the vapor exerts a low pressure in a closed container. Low vapor pressure is an important physical property of water, since lakes, oceans, and other large bodies of water would all tend to evaporate much more quickly otherwise.
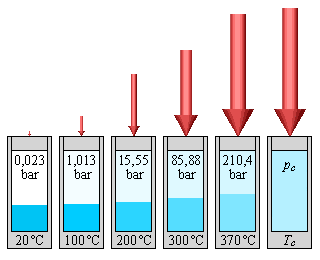
::液体蒸发压是封闭容器中液体或固体蒸发产生的蒸发压,液体或固体在液体或固体之上产生的蒸发压,液体水分子之间的氢联结解释了为什么水的含量非常低。相对较少的水分子能够逃离液体表面进入蒸发阶段。蒸发速度缓慢,因此蒸发器在封闭容器中施加低压。低蒸发压是水的重要物理特性,因为湖泊、海洋和其他大水体都倾向于更快地蒸发。Vapor pressure is influenced by temperature. As the temperature increases, more molecules are released from the surface of the liquid. This increases movement above the liquid surface, increasing the pressure in the vapor stage. The image below illustrates the on vapor pressure.
::蒸汽压力受温度影响。 随着温度的升高, 液体表面释放出更多的分子。 这会增加液体表面的移动, 增加蒸汽阶段的压力。 下面的图像显示蒸汽压力。Science Friday: Candy Corn in Space
::科学星期五:太空糖果玉米Candy corn is a very tasty treat. In this video by Science Friday, astronaut Don Pettit uses Candy Corn to demonstrate the effects of hydrophobic and hydrophilic interactions.
::玉米糖果是一个非常美味的乐谱。在这个由科学星期五拍摄的视频中,宇航员唐·佩蒂特利用玉米糖果展示了疏水和水利相互作用的影响。Water molecules are really special. Try out this simulation and see how they interact:
::水分子是非常特殊的。尝试一下这个模拟,看看它们是如何相互作用的:Summary
::摘要-
Water has high surface tension because of extensive hydrogen bonding.
::由于氢气的广泛结合,水的表面高度紧张。 -
The vapor pressure of water is low due to hydrogen bonding.
::由于氢结合,水的蒸气压较低。 -
Vapor pressure increases as temperature increases.
::蒸气压力随着温度升高而增加。
Review
::回顾-
What is surface tension?
::表面紧张是什么? -
What is vapor pressure?
::什么是蒸气压? -
How does temperature affect vapor pressure?
::温度如何影响蒸气压力?
-
Water has high surface tension because of extensive hydrogen bonding.