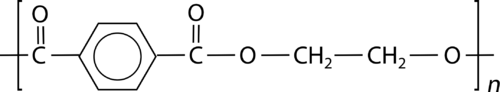聚化 - 凝聚聚聚聚体
章节大纲
-
Why is there a different sound and feel?
::为什么有不同的声音和感觉?Animal gut and silk were used for all guitar strings for centuries until modern technology and changes in musical taste brought about significant changes. There are two major types of guitar strings in use today. Steel strings (first developed around 1900) are found on acoustic and electric guitars. They have a bright, crisp sound that lends itself well to diverse music as jazz, rock and roll, and bluegrass. Nylon strings are a more recent development. During World War II, the silk and animal products needed to manufacture steel guitar strings were not available. Nylon quickly proved to be a more than adequate substitute. Now nylon strings are found on all classical guitars. Their sound is somewhat softer than the steel strings and the tone quality suits that genre of music better.
::几个世纪以来,所有吉他琴弦都使用了动物肠和丝绸,直到现代技术和音乐品味的变化带来重大变化。今天使用的吉他弦有两大类。钢弦(最初开发于1900年左右)在音响和电动吉他上。钢弦(最初开发于1900年左右)在音响和电动吉他上找到。它们有一个亮亮亮的、响亮的音响,它非常适合爵士、摇滚和蓝草等多种音乐。尼龙弦是最近的一种发展。在第二次世界大战期间,没有制造钢吉他弦所需的丝和动物产品。尼龙很快被证明是一个更充分的替代物。现在,尼龙弦在所有古典吉他上都找到了。它们的声音比钢弦和音质优于音乐的音质的更柔软一些。Polymerization - Condensation Polymers
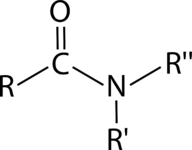
::聚化 - 凝聚聚聚聚体A condensation polymer is a polymer formed by reactions. Monomers of condensation polymers must contain two functional groups so that each monomer can link up with two other monomers. One type of condensation polymer is called a polyamide. An amide is characterized by the functional group shown below wherein the carbon of a carbonyl group is bonded to the nitrogen of an .
::凝聚聚合物是一种由反应形成的聚合物。 凝聚聚合物的单体必须包含两个功能组,这样每个单体都能够与其他两个单体相连。 一种凝聚聚合物称为聚酰胺。 氨化物的特征为以下显示的功能组,其中碳基组的碳与一个碳的氮结合。Amide
::氨One pair of monomers that can form a polyamide is adipic and hexanediamine. Adipic acid is a carboxylic acid with two carboxyl groups on either end of the molecule. Hexanediamine has amino groups on either end of a six-carbon chain. When these molecules react with each other, a molecule of water is eliminated, classifying it as a condensation reaction (see Figure ).
::一种可形成聚酰胺的单体是二甲胺和六硝胺。二甲酸是一种箱状酸,分子两端都有两个碳苯基组。六乙烷胺在六碳链两端都有氨基组。当这些分子相互反应时,就会消除水分子,将其分类为凝聚反应(见图 )。Nylon synthesis
::尼隆综合合成The polymer that results from the repetition of the condensation reaction is a polyamide called nylon-66. Nylon-66 was first invented in 1935 and has been used in all sorts of products. It and other polyamides are commonly found in fibers and clothing, cooking utensils, fishing line, and carpeting, among many other applications.
::Nylon-66是1935年首次发明的,用于各种产品,其纤维和衣服、烹饪用具、捕鱼线和地毯等许多应用中通常都有这种聚合物和其他聚酰胺。Nylon spatula.
::尼龙斯波图拉Polyester is another common type of condensation polymer. Recall that are formed from the reaction of an with a carboxylic acid. When both the acid and alcohol have two functional groups, the ester is capable of being polymerized. One such polyester is called polyethylene terephthalate (PET) and is formed from the reaction of ethylene glycol with terephthalic acid. The structure of PET is shown below.
::聚酯是另一种常见的凝聚聚合物,召回由一种与碳酸反应形成,当酸和酒精有两种功能组时,该酯就能够聚合,其中一种聚酯称为聚乙烯甲硫二甲酸(PET),由乙基甘醇与磷酸反应形成,如下表所示。PET is used in tires, photographic film, food packaging, and clothing. Polyester fabric is used in permanent-press clothing. Its resistance to wrinkling comes from the cross-linking of the polymer strands.
::PET用于轮胎、照相胶片、食品包装和服装,聚酯织物用于永久压质服装,其耐皱性来自聚合物束的交叉连接。Review
::回顾-
What is a condensation reaction?
::什么是凝聚反应? -
What structural characteristics do the two monomers need to have?
::两个单一体需要具备哪些结构特征? -
What monomers compose nylon-66?
::尼龙 -66是什么单体?
-
What is a condensation reaction?