国家变更
章节大纲
-
Before the was invented, steam engines were the power source for ships, locomotives, tractors, lumber saws, and most industrial machines. Coal or wood was burned to boil water into steam, which ran the engine.
::在发明之前,蒸汽发动机是船舶、机车、拖拉机、木材锯和大多数工业机器的动力源。 煤或木材被烧焦,将水煮成蒸汽,而蒸汽是发动机的动力源。Change of State
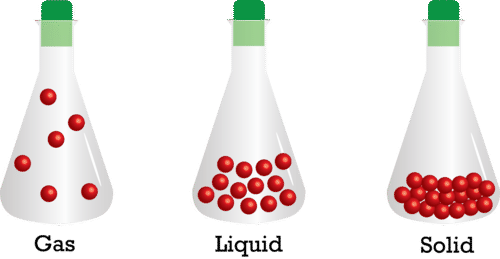
::国家变更Most substances may exist in any of the three common states of matter. In the gaseous state, the molecular motion has completely overcome any attraction between the particles and the particles are totally separate from each other. There are large spaces between the particles and they move large distances between collisions. In the liquid state, the molecular motion and the molecular attractions are more balanced. While the particles stay more or less in contact with each other, they are still free to move and can slide past one another easily. In the solid state, the attractive forces dominate. The particles are pulled together into a tightly packed pattern which does not allow the particles to pass each other. The molecular motion in this form is essentially reduced to vibration in place. Increasing the of a substance means increasing the molecular motion (kinetic energy) of the molecules in the substance. The phase in which a substance exists is the result of a competition between attractive forces and molecular motion.
::大多数物质可能存在于三个共同物质状态中的任何状态中。 在气体状态中, 分子运动已经完全克服了粒子之间的任何吸引力, 粒子之间是完全分离的。 粒子之间有较大的空间, 它们相撞之间有很远的距离。 在液体状态中, 分子运动和分子吸引比较平衡。 虽然粒子保持或多或少地相互接触, 它们仍然可以自由移动, 可以很容易地滑过。 在固体状态中, 吸引的力量支配着它们。 粒子被拉在一起, 形成一个紧凑的包装模式, 不允许粒子相互传递。 以这种形式出现的分子运动基本上被减为振动。 物质增加意味着物质中分子的分子运动( 动能) 。 物质存在的阶段是有吸引力的力量和分子运动之间竞争的结果。For most substances, when the temperature of the solid is raised high enough, the substance changes to a liquid, and when the temperature of the liquid is raised high enough, the substance changes to a gas. We typically visualize a solid as tiny particles in constant motion held together by attractive forces. As we add to the solid, the motion, or the , of the particles increases. At some temperature, the motion of the particles becomes great enough to overcome the attractive forces. The that was added to the solid up to this point was absorbed by the solid as kinetic energy, increasing the of the molecules. The lowest temperature at which the particles are able to exist in the liquid form is called the melting point .
::对于大多数物质来说,当固体温度升高到足够高时,物质会改变到液体,当液体温度升高到足够高时,物质会改变到气体。我们通常会把固体想象成微粒,在有吸引力的动力下,恒定运动在一起。当我们把微粒增加到固体时,运动或粒子的增加。在某种温度下,粒子的运动变得足够大,足以克服有吸引力的力量。在这一点上添加到固体的微粒被固体吸收,作为动能,增加分子。微粒以液体形式存在的最低温度被称为熔点。In order for the molecules to actually separate from each other, more must be added. This energy, called heat of fusion or heat of melting, is absorbed by the particles as as the solid changes to a liquid. Recognize that, once the temperature of a solid has been raised to the melting point, it is still necessary for the solid to absorb additional thermal energy in the form of potential energy as the molecules separate.
::为了让分子实际上彼此分离,必须增加更多。这种能量被称为聚变热或熔化热,作为液体的固态变化被颗粒吸收。认识到一旦固体温度升高到熔点,固体仍有必要吸收额外的热能,即分子分离时的潜在能量。The boiling point of a liquid is the temperature at which the particles have sufficient molecular motion to exist in the form of a gas. Once again, however, in order for the particles to separate to the gaseous form, they must absorb a sufficient amount of potential energy. The amount of potential energy necessary for a phase change to gaseous form is called the heat of vaporization . Consider the heating curve shown below.
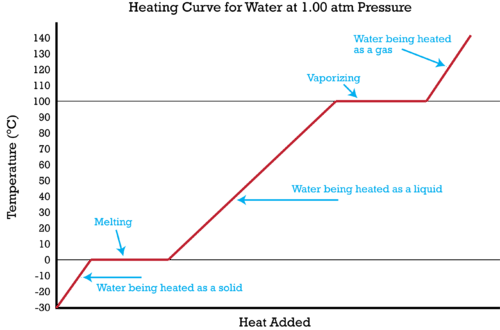
::液体的沸点是粒子有足够的分子运动以气体形式存在的温度。然而,为了让粒子与气体形式分离,它们必须吸收足够的潜在能量。逐步改变气体形式所需的潜在能量量称为蒸发热。请参考下面显示的加热曲线。The heating curve shown is for water but other substances have similarly shaped heating curves. Suppose you begin with solid water (ice) at -30°C and add heat at a constant rate. The heat you add in the beginning will be absorbed as kinetic energy and the temperature of the solid will increase. When you reach a temperature of 0°C (the melting point for water), the heat you add is no longer absorbed as kinetic energy. Instead, the added heat is absorbed as potential energy and the particles separate from each other. During the flat part of the curve labeled “melting”, heat is being added constantly but the temperature does not increase. At the left edge of this flat line, the water is solid; by the time enough heat has been added to get to the right edge, the water is liquid, but maintains the same temperature. Once all the water is in the liquid form, the added heat will once again be absorbed as kinetic energy and the temperature will increase again. During the time labeled “water being heated as a liquid”, all the added heat is absorbed as kinetic energy.
::显示的加热曲线是水的, 但其他物质有相似的制热曲线。 假设您在 - 30 °C 时以固态水( 冰) 开始, 并以恒定速度添加热量。 开始时添加的热量将随着动能吸收, 固体温度将升高。 当温度达到0°C( 水的熔点) 时, 您添加的热量将不再被吸收为动能。 相反, 添加的热量被吸收为潜在的能量, 而粒子会彼此分离。 在标有“ 熔化” 的曲线平方部分, 热量会不断增加, 但温度不会增加 。 在这条平线的左边缘, 水是固体的; 等到足够热量被添加到右边缘时, 水是液体, 但保持同样的温度。 一旦所有水在液体中, 添加的热量将再次被吸收为动能, 温度将再次升高。 在标有“ 水被加热量作为液体” 时, 所有添加的热量都会被吸收为动能 。When a temperature of 100°C (the boiling point of water) is reached, the added heat is once again absorbed as potential energy and the molecules separate from liquid form into gaseous form. When all the substance has been converted into gas, the temperature will again begin to rise. Use the simulation below to further explore the heating curve for water and visualize the how water changes at the molecular level during each phase illustrated in the graph:
::当温度达到100°C(水的沸点)时,添加的热再次被吸收为潜在能量,而分子则与液体形式分开,形成气体形态。当所有物质被转化成气体时,温度将再次上升。使用下面的模拟来进一步探索水的加热曲线,并直观地显示在图所示的每个阶段的分子层次上水的变化:Heats of Fusion and Vaporization of Some Common Substances Substance Heat of Fusion, Heat of Vaporization, Copper Gold Iron Methanol Water When the temperature of a substance is changing, we can use the to determine the amount of heat that is being gained or lost. When a substance is changing phase, we can use the heat of fusion or heat of vaporization to determine the amount of heat being gained or lost. When a substance freezes from liquid to solid, the amount of heat given off is exactly the same as the amount of heat absorbed when the substance melts from solid to liquid. The equations for heat gained or lost are given here:
::当物质温度发生变化时,我们可以使用该物质来确定正在获取或丧失的热量。当一种物质正在变化阶段时,我们可以使用聚变热或蒸发热来确定热量的增减。当一种物质从液体冻结到固体时,所释放的热量与物质从固体熔化到液体时所吸收的热量完全相同。这里给出的热增减的方程式如下:The heat gained or lost during a temperature change: .
::温度变化期间的热量增减:@mct。The heat gained or lost during a phase change of solid to liquid: .
::固态改为液态的阶段变暖过程中的热量增益或损耗:mHf。The heat gained or lost during a phase change of liquid to gas: .
::液态转化为气体的阶段变换过程中产生的或损失的热量:++mHv。Examples
::实例Example 1
::例15000 Joules of heat is added to ice at 273 K. All the heat goes into changing solid ice into liquid water. How much ice is melted?
::五千焦耳的热量在273千兆赫的冰中添加。所有的热量都流入将固体冰变成液态水。有多少冰被融化了?
::mHf=500 J3.34×105 J/kg=0.0150公斤Example 2
::例2Beginning with 1.00 kg of ice at -20.0°C, heat is added until the substance becomes water vapor at 130.0°C. How much heat was added? The specific heat of ice is , the specific heat of liquid water is , and the specific heat of water vapor is .
::从摄氏-20.0摄氏度时的1.00千克冰开始,加热直至该物质在130.0摄氏度时成为水蒸气。加热多少?冰的具体热量是2108 J/kgQ°C,液态水的具体热量是4187 J/kgQ°C,而水蒸气的具体热量是1996 J/kgQ°C。5 steps:
::5个步骤:-
Calculate the heat required to raise the sample from -20.0°C to 0°C.
::计算将样品从-20.0°C升至-0°C所需的热量。 -
Calculate the heat required to melt the sample.
::计算熔化样品所需的热量。 -
Calculate the heat required to raise the sample from 0°C to 100°C.
::计算将样品从0°C升至100°C所需的热量。 -
Calculate the heat required to vaporize the sample.
::计算蒸发样本所需的热量。 -
Calculate the heat required to raise the sample from 100°C to 130°C.
::计算将样品从100°C升至130°C所需的热量。
The solution is the sum of these steps.
::解决办法就是这些步骤的总和。
::1.QHS=mcicet=(1千克)(1,00千克)(2108 J/kg.C)(20.0C)=42160 J
::2. QMelt=mHf=(1.00公斤)(33400日/公斤)=33400日/公斤)=33400日
::3.QHL=mcwatert=(1.00千克)(4187 J/kg.C)(1000.0C)=41.8700 J)
::4.Vap=mHv=(1.00千克)(2260000 J/kg)=2260000 J
::5.HV=mcvaport=1.00公斤(1996年J/kg.C)(30.0C)=59880焦耳
::热总量=3.11×106 JSummary
::摘要-
Most substances may exist in any of the three common states of matter, solid, liquid, or gas.
::大多数物质可能存在于三个共同的物质状态中的任何一种,即固体、液体或气体。 -
The phase in which a substance exists is the result of a competition between attractive forces and molecular motion.
::物质存在的阶段是具有吸引力的力量和分子运动之间竞争的结果。 -
The potential energy absorbed by a solid as it changes to a liquid is called the heat of fusion or the heat of melting.
::固体在改变液体时吸收的潜在能量称为聚变热或熔化热。 -
The amount of potential energy necessary for a phase change to gaseous form is called the heat of vaporization.
::逐步改变气态所需的潜在能量量称为蒸发热。 -
The heat gained or lost during a temperature change is given by,
.
::温度变化中产生的或损失的热量由 mct 给出。 -
The heat gained or lost during a phase change of solid to liquid is given by,
.
::固态转化为液体的阶段变暖过程中产生的或损失的热量由 mHf提供。 -
The heat gained or lost during a phase change of liquid to gas is given by,
.
::液态转化为气体的阶段变换过程中产生的或损失的热量由 mHv 提供。
Review
::回顾-
A 200 g sample of water at 60.0°C is heated to water vapor at 140.0°C. How much heat was absorbed?
::在 6 0.0 °C 6 0.0 °C 的200 克水样本在 14 0.0 °C 的 14 0.0 °C 时加热为水蒸气。 吸收了多少热量? -
A 175 g lump of molten lead at its melting point (327°C) is placed into 55.0 g of water at 20.0°C. The specific heat of lead is
and the
of lead is 20,400 J/kg.
-
When the lead has become solid but is still at the melting point, what is the temperature of the water?
::当铅变得固体,但仍处于熔点时,水的温度是多少? -
When the lead and the water have reached equilibrium, what is the temperature of the mixture?
::当铅和水达到平衡时,混合物的温度是多少?
::熔点(327°C)的175克熔化铅块在20.0°C时被放入55.0克的水中。铅的具体热量为130 J/kgC,铅的热量为20,400 J/kg。当铅变得固体但仍处于熔点时,水的温度是多少?当铅和水达到平衡时,混合物的温度是多少? -
When the lead has become solid but is still at the melting point, what is the temperature of the water?
Explore More
::探索更多Use this video to answer the questions that follow.
::使用这段视频回答接下来的问题。-
For water, which takes more energy, melting or evaporating?
::水需要更多能量 熔化还是蒸发? -
When are there two phases present at the same time in the pot?
::罐子中何时同时存在两个阶段?
-
Calculate the heat required to raise the sample from -20.0°C to 0°C.