物质动态理论
章节大纲
-
This neat row of cola bottles represents matter in three different states— , , and . The bottles and caps are solids, the cola is a liquid, and carbon dioxide dissolved in the cola is a gas. It gives cola its fizz. Solids, liquids, and gases such as these have different properties. Solids have a fixed shape and a fixed volume . Liquids also have a fixed volume but can change their shape. Gases have neither a fixed shape nor a fixed volume. What explains these differences in ? The answer has to do with .
::这排整齐的可乐瓶子代表了三个不同的状态中的物质。 瓶子和瓶盖是固态的, 可乐是液态的, 而可乐中溶解的二氧化碳是气态的。 它给出了可乐的纤维。 固体、 液体和气体有不同的特性。 固体有固定的形状和固定的体积。 液体也有固定的体积, 但可以改变形状。 气体没有固定的形状, 也没有固定的体积。 答案与这些区别有关 ?Moving Matter
::移动事项Energy is the ability to cause changes in matter. For example, your body uses chemical energy when you lift your arm or take a step. In both cases, energy is used to move matter—you. Any matter that is moving has energy just because it’s moving. The energy of moving matter is called . Scientists think that the particles of all matter are in constant motion. In other words, the particles of matter have kinetic energy. The theory that all matter consists of constantly moving particles is called the .
::能量是改变物质的能力。例如,你的身体在举起手臂或一步步时使用化学能量。在这两种情况下,能量都用来移动物质——你。任何移动的事物都有能量,只是因为它在移动。移动物质的能量被称为移动物质的能量。科学家认为所有物质的粒子都在不断移动。换句话说,物质粒子有动能。所有物质都包含不断移动的粒子的理论都被称为移动粒子。Kinetic Energy and States of Matter
::动能和物质国Differences in kinetic energy explain why matter exists in different states. Particles of matter are attracted to each other, so they tend to pull together. The particles can move apart only if they have enough kinetic energy to overcome this force of attraction. It’s like a tug of war between opposing sides, with the force of attraction between particles on one side and the kinetic energy of individual particles on the other side. The outcome of the “war” determines the state of matter.
::电动能量的差异解释了为什么物质存在于不同的州。 物质粒子相互吸引,因此它们往往合在一起。 粒子只有在有足够的动能来克服这种吸引力的情况下才能分离。 这就像对立方之间的一场拖拉式战争,一方面是粒子的吸引力,另一方面是个别粒子的动能。 “ 战争”的结果决定了物质状态。-
If particles do not have enough kinetic energy to overcome the force of attraction between them, matter exists as a solid. The particles are packed closely together and held rigidly in place. All they can do is vibrate. This explains why solids have a fixed volume and a fixed shape.
::如果粒子没有足够的动能来克服它们之间的吸引力, 物质会以固体的形式存在。 粒子会紧密地包装在一起, 并僵硬地固定在原地。 它们所能做的就是振动。 这就解释了为什么固体有固定的体积和固定的形状。 -
If particles have enough kinetic energy to partly overcome the force of attraction between them, matter exists as a liquid. The particles can slide past one another but not pull apart completely. This explains why liquids can change shape but have a fixed volume.
::如果粒子有足够的动能来部分克服它们之间的吸引力, 物质就作为液体存在。 粒子可以相互滑过, 但不能完全分离。 这就解释了为什么液体可以改变形状, 但却有一个固定的体积 。 -
If particles have enough kinetic energy to completely overcome the force of attraction between them, matter exists as a gas. The particles can pull apart and spread out. This explains why gases have neither a fixed volume nor a fixed shape.
::如果粒子有足够的动能来完全克服它们之间的吸引力,物质就作为一种气体存在。粒子可以分离和扩散。这解释了为什么气体既没有固定的体积,也没有固定的形状。
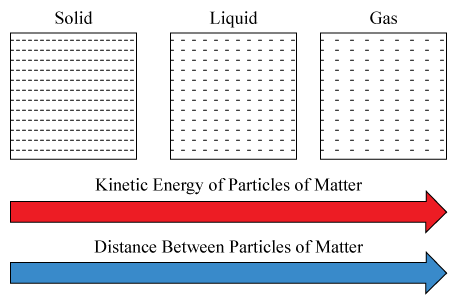
Look at the Figure . It sums up visually the relationship between kinetic energy and state of matter.
::看看图。它从视觉上总结了动能和物质状态之间的关系。Q: How could you use a bottle of cola to demonstrate these relationships between kinetic energy and state of matter?
::问题:你如何使用一瓶可乐来证明动能和物质状态之间的关系?A: You could shake a bottle of cola and then open it. Shaking causes carbon dioxide to come out of the cola solution and change to a gas. The gas fizzes out of the bottle and spreads into the surrounding air, showing that its particles have enough kinetic energy to spread apart. Then you could tilt the open bottle and pour out a small amount of the cola on a table, where it will form a puddle. This shows that particles of the liquid have enough kinetic energy to slide over each other but not enough to pull apart completely. If you do nothing to the solid glass of the cola bottle, it will remain the same size and shape. Its particles do not have enough energy to move apart or even to slide over each other.
::甲:你可以摇一瓶可乐,然后打开它。 摇动会使二氧化碳从可乐溶液中流出, 变成气体。 瓶中气雾, 扩散到周围空气中, 表明其粒子有足够的动能分散。 然后你可以倾斜开瓶, 将少量可乐倒在桌上, 在那里它会形成一个水坑。 这表明液体的粒子有足够的动能可以滑过对方, 但不足以完全分离。 如果您对可乐瓶的固体玻璃不做任何反应, 它的大小和形状将保持不变。 它的粒子没有足够的能量分开甚至滑过对方。Summary
::摘要-
According to the kinetic theory, particles of matter are in constant motion. The energy of motion is called kinetic energy.
::根据动能理论,物质粒子在不断运动。运动的能量被称为动能。 -
Particles of solids have the least kinetic energy and particles of gases have the most.
::固体粒子的动能最低,气体粒子最多。
Review
::回顾-
Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor.
::使用动能分子物质理论来描述冰、液态水和水蒸气中的粒子运动。 -
What is the relationship between the kinetic energy of particles and the forces of attraction between particles?
::粒子动能与粒子之间吸引力之间的关系是什么?
Explore More
::探索更多Watch the video below and then answer the questions that follow.
::观看下面的录像,然后回答下面的问题。-
Describe the motion of particles in ice, liquid water, and water vapor.
::描述冰、液态水和水蒸气中的粒子运动。 -
Apply the kinetic theory of matter to explain the differences in your answer to question 1.
::运用动态物质理论来解释你对问题1的答复中的差异。
-
If particles do not have enough kinetic energy to overcome the force of attraction between them, matter exists as a solid. The particles are packed closely together and held rigidly in place. All they can do is vibrate. This explains why solids have a fixed volume and a fixed shape.