汤姆森原子模型
章节大纲
-
You probably know that the wires strung between these high towers carry electricity. But do you know what electricity is? It actually consists of a constant stream of tiny particles called electrons. Electrons are negatively charged inside atoms. Atoms were discovered around 1800, but almost 100 years went by before electrons were discovered.
::你可能知道这些高塔之间的电线是电的。但你知道电是什么吗?它实际上包括一条称为电子的恒定微粒流。电子在原子中受到负电荷。原子在1800年左右被发现,但在发现电子之前已经过了近100年。Thomson Discovers Electrons
::汤姆森发现电子John Dalton discovered atoms in 1804. He thought they were the smallest particles of matter, which could not be broken down into smaller particles. He envisioned them as , hard spheres. It wasn’t until 1897 that a scientist named Joseph John (J. J.) Thomson discovered that there are smaller particles within the . Thomson was born in England and studied at Cambridge University, where he later became a professor. In 1906, he won the Nobel Prize in physics for his research on how conduct electricity. This research also led to his discovery of the . You can see a picture of Thomson .
::1804年,约翰·道尔顿发现了原子。他认为这些微粒是最小的物质粒子,无法细分为较小的粒子。他把它们想象成硬球。直到1897年,一个名叫约瑟夫·约翰(J.J.)的科学家汤姆森才发现,在...汤姆森(Joseph John,J.J.)体内有小粒子。汤姆森出生在英国,在剑桥大学学习,后来在剑桥大学成为教授。1906年,他获得诺贝尔物理奖,以研究如何进行电力。这项研究还导致他发现了这些微粒。你可以看到汤姆森的一张照片。Thomson’s Experiments
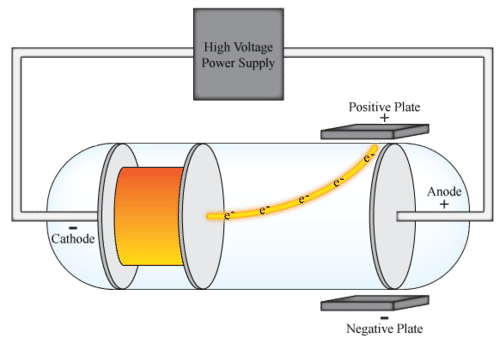
::汤姆森的实验In his research, Thomson passed current through a cathode ray tube, similar to the one seen in the Figure . A cathode ray tube is a glass tube from which virtually all of the air has been removed. It contains a piece of metal called an electrode at each end. One electrode is negatively charged and known as a cathode. The other electrode is positively charged and known as an anode. When high- voltage electric current is applied to the end plates, a cathode ray travels from the cathode to the anode.
::在其研究中,Thomson通过阴极射线管通过电流通过阴极射线管,类似于图中看到的阴极射线管。阴极射线管是一个玻璃管,几乎所有空气都已经从这个管中移除。它包含一个金属块,叫做每个端的电极。一个电极是负电的,被称为阴极。另一个电极是正电,称为阳极。当对末端板应用高压电流时,阴极射线线从阴极到阳极。What is a cathode ray? That’s what Thomson wanted to know. Is it just a ray of that travels in waves like a ray of light? That was one popular at the time. Or was a cathode ray a stream of moving particles? That was the other popular hypothesis. Thomson tested these ideas by placing negative and positive plates along the sides of the cathode ray tube to see how the cathode ray would be affected. The cathode ray appeared to be repelled by the negative plate and attracted by the positive plate. This meant that the ray was negative in charge and that is must consist of particles that have mass. He called the particles “corpuscles,” but they were later renamed electrons.
::阴极射线是什么?Thomson想知道的是什么?Thomson想要知道的就是阴极射线。它只是波浪中的一线光线,像光线般的光线吗?它当时很流行吗?还是阴极射线是移动颗粒的流?这是另一个流行的假设。Thomson在阴极射线管的两侧放置阴和阳性板来测试这些想法,看阴极射线将如何受到影响。阴极射线似乎被负盘反射,被正面吸引。这意味着阴极射线是负载的,必须由有质量的粒子组成。他称这些粒子为“细胞 ” , 但它们后来更名为电子。Thomson also measured the mass of the particles he had identified. He did this by determining how much the cathode rays were bent when he varied the voltage. He found that the mass of the particles was 2000 times smaller than the mass of the smallest atom, the hydrogen atom. In short, Thomson had discovered the existence of particles smaller than atoms. This disproved Dalton’s claim that atoms are the smallest particles of matter. From his discovery, Thomson also inferred that electrons are fundamental particles within atoms.
::汤姆森还测量了他所发现的粒子的质量。 他这样做的方法是确定在他改变电压时阴极射线弯曲了多少。 他发现这些粒子的质量比最小原子即氢原子的质量小2000倍。 简而言之,汤姆森发现了小于原子的粒子的存在。 这推翻了道尔顿关于原子是最小物质粒子的说法。 从他的发现来看,汤姆森还推断电子是原子中的基本粒子。Q: Atoms are neutral in electric charge . How can they be neutral if they contain negatively charged electrons?
::问题:原子在电荷中是中性的。如果原子含有负电荷电子,它们怎么能是中性的呢?A: Atoms also contain positively charged particles that cancel out the negative charge of the electrons. However, these positive particles weren’t discovered until a couple of decades after Thomson discovered electrons.
::A:原子还含有正电荷粒子,消除了电子的负电荷。 然而,这些正粒子直到汤姆森发现电子几十年后才被发现。The Plum Pudding Model
::普拉姆布布料模型Thomson also knew that atoms are neutral in electric charge, so he asked the same question: How can atoms contain negative particles and still be neutral? He hypothesized that the rest of the atom must be positively charged in order to cancel out the negative charge of the electrons. He envisioned the atom as being similar to a plum pudding, like the one pictured in the Figure —mostly positive in charge (the pudding) with negative electrons (the plums) scattered through it.
::汤姆森还知道原子在电荷中是中性的,因此他问了同样的问题:原子怎么会含有负粒子,并且仍然保持中性?他假设原子的其余部分必须被正面电荷,才能取消电子的负电荷。 他认为原子类似于梅布丁,就像图中描绘的那样 — — 主要是负电荷(布丁)正对,其中含有分散在其中的负电子(羽子)。Q: How is our modern understanding of atomic structure different from Thomson’s plum pudding model ?
::问:我们现代对原子结构的理解与汤姆森的梅布丁模型有何不同?A: Today we know that all of the positive charge in an atom is concentrated in a tiny central area called the nucleus , with the electrons swirling through empty space around it, as in the Figure . The nucleus was discovered just a few years after Thomson discovered the electron, so the plum pudding model was soon rejected.
::A:今天,我们知道原子中的所有正电荷都集中在一个叫做核的微小中央区域,核心被称作核,电子在它的周围的空隙中旋转,如图中所示。这个核是在汤姆森发现电子之后仅仅几年才发现的,因此梅布丁模型很快被否决了。Summary
::摘要-
In 1897, J. J. Thomson discovered the first subatomic particle, the electron, while researching cathode rays.
::1897年,J.J.汤姆森在研究阴极射线时发现了第一个亚原子粒子,即电子。 -
To explain the neutrality of atoms, Thomson proposed a model of the atom in which negative electrons are scattered throughout a sphere of positive charge. He called his atom the plum pudding model.
::为了解释原子的中立性,汤姆森提出了一个原子模型,负电子散布在正电荷范围内。他称其原子为梅布布丁模型。
Review
::回顾-
Who was J. J. Thomson?
::谁是J. J. 汤姆森? -
Explain how Thomson discovered negatively charged particles smaller than atoms.
::解释一下汤姆森是如何发现负电荷粒子 小于原子。 -
Thomson compared his idea of atomic structure to a plum pudding. Invent an original analogy for Thomson’s plum pudding model of the atom.
::汤姆森将原子结构的理念比作梅布丁。 发明了汤姆森原样的原子梅布丁模型的原始比喻。 -
Why was Thomson’s model soon rejected?
::为什么汤姆森的模型很快被否决?
Explore More
::探索更多Watch this detailed presentation about J. J. Thomson’s discovery of the electron, and then answer the question below.
::观察J. J. Thomson对电子发现的详细介绍, 然后回答下面的问题。-
Thomson not only discovered that a cathode ray consists of flowing negatively charged particles that are smaller than atoms. He also made the logical leap that these particles help make up atoms. What reasoning did Thomson use to make this inference?
::汤姆森不仅发现阴极射线是由小于原子的负电荷粒子组成的。 他还做出了这些粒子帮助原子组成的逻辑飞跃。 汤姆森用什么推理来推断呢?
-
In 1897, J. J. Thomson discovered the first subatomic particle, the electron, while researching cathode rays.