非金属
章节大纲
-
The three pure substances pictured above have the distinction of being among the top ten that make up the human body. All three of them belong to the class of elements called nonmetals. Most of the elements that comprise the human body—as well as the majority of other living things—are nonmetals. In fact, seven of the top ten elements in your own body belong to this class of elements. What do you know about nonmetals? What are their properties, and how are they different from other elements? In this article, you’ll find out.
::上面描述的三种纯物质具有与构成人体的前十种物质之间的区别,所有三种物质都属于称为非金属的元素。构成人体的多数元素,以及其他大多数生物物质,都是非金属的元素。事实上,你身体中前十种元素中的七种元素中,有七种元素属于这种元素。你对非金属有什么了解?它们有哪些特性,它们与其他元素有何不同?在本篇文章中,你会发现它们与其他元素有何不同。What Are Nonmetals?
::什么是非金属?Nonmetals are elements that generally do not conduct electricity. They are one of three classes of elements (the other two classes are and .) Nonmetals are the second largest of the three classes after metals. They are the elements located on the right side of the periodic table .
::非金属是通常不进行电力的元素,是三种元素中的一种(其他两类为和.)。 非金属是金属之后三种元素中第二大元素,是周期表右侧的元素。Q: From left to right across each period (row) of the periodic table, each element has atoms with one more and one more than the element before it. How might this be related to the properties of nonmetals?
::问题:在周期表的每个周期(行)中,从左到右,每个元素都有原子,其面前的元素是一个以上和一个以上。这与非金属的特性有何关系?A: Because nonmetals are on the right side of the periodic table, they have more electrons in their outer than elements on the left side or in the middle of the periodic table. The number of electrons in the outer energy level of an determines many of its properties.
::甲:由于非金属位于周期表的右侧,其外表的电子量比左侧或周期表中间的元素多。一个外能水平中的电子数量决定其许多特性。Properties of Nonmetals
::非金属特性As their name suggests, nonmetals generally have properties that are very different from the properties of metals. Properties of nonmetals include a relatively low point, which explains why many of them are at room . However, some nonmetals are solids at room temperature, including the three pictured above, and one nonmetal—bromine—is a at room temperature. Other properties of nonmetals are illustrated and described in the Figure .
::如其名称所示,非金属的特性通常与金属的特性大不相同,非金属的特性包括一个相对较低的点,这解释了许多非金属在室内的原因。然而,有些非金属在室内温度下是固体,包括上面的三幅图,一个非金属-溴-在室内温度下是固体。图中说明了非金属的其他特性。Reactivity of Nonmetals
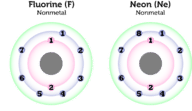
::非金属反应Reactivity is how likely an element is to react chemically with other elements. Some nonmetals are extremely reactive, whereas others are completely nonreactive. What explains this variation in nonmetals? The answer is their number of . These are the electrons in the outer energy level of an atom that are involved in interactions with other atoms. Let’s look at two examples of nonmetals, fluorine and neon. Simple atomic models of these two elements are shown in the Figure .
::反应是元素与其他元素发生化学反应的可能性。有些非金属极具反应性,而另一些非金属则完全无反应性。解释非金属中这种变化的原因是什么?答案是它们的数量。这些是原子外能层中与其他原子相互作用的原子外能层中的电子。让我们看看非金属、氟和纳米的两个例子。图中显示了这两个元素的简单原子模型。Q: Which element, fluorine or neon, do you predict is more reactive?
::问题:什么元素, 氟或荧光, 你是否预测更具有反应性?A: Fluorine is more reactive than neon. That’s because it has seven of eight possible electrons in its outer energy level, whereas neon already has eight electrons in this energy level.
::甲:氟比荧光更具反应性。 这是因为它外部能源水平有八种电子中的七种,而在这种能量水平上有八种电子。Although neon has just one more electron than fluorine in its outer energy level, that one electron makes a huge difference. Fluorine needs one more electron to fill its outer energy level in order to have the most stable arrangement of electrons. Therefore, fluorine readily accepts an electron from any element that is equally “eager” to give one up, such as the metal lithium or sodium. As a result, fluorine is highly reactive. In fact, reactions with fluorine are often explosive. Neon, on the other hand, already has a full outer energy level. It is already very stable and never reacts with other elements. It neither accepts nor gives up electrons. Neon doesn’t even react with fluorine, which reacts with all other elements except helium.
::尽管氢在外能水平上只拥有比氟更多的一个电子,但一种电子会产生巨大的不同。 氟需要多一个电子来填充其外能水平,以便拥有最稳定的电子配置。 因此,氟能很容易地接受任何同样“渴望”放弃一个元素的电子,例如金属锂或钠。结果,氟反应性很强。事实上,对氟的反应往往是爆炸性的。 另一方面,尼昂已经拥有一个完整的外能水平。它已经非常稳定,从不与其他元素发生反应。它既不接受也不放弃电子。 尼昂甚至不会对氟反应,因为氟对所有其他元素的反应都是例外的。Why Most Nonmetals Cannot Conduct Electricity
::为何多数非金属无法发电Like most other nonmetals, fluorine cannot conduct electricity, and its electrons explain this as well. An electric current is a flow of electrons. Elements that readily give up electrons (the metals) can carry electric current because their electrons can flow freely. Elements that gain electrons instead of giving them up cannot carry electric current. They hold onto their electrons so they cannot flow.
::与大多数其他非金属一样,氟不能进行电力,其电子也解释这一点。电流是电子流动。 很容易放弃电子(金属)的元素可以携带电流,因为其电子可以自由流动。 获得电子而不是放弃电子的元素不能携带电流。 它们将电流握在电子上,因此无法流动。Summary
::摘要-
Nonmetals are elements that generally cannot conduct electricity. They are the second largest class of elements after metals. Examples of nonmetals include hydrogen, carbon, chlorine, and helium.
::非金属是一般无法进行电力的元素,是金属之后第二大元素,非金属的例子包括氢、碳、氯和。 -
Properties of nonmetals include a relatively low boiling point, so many nonmetals are gases. Nonmetals are also poor conductors of heat, and solid nonmetals are dull and brittle.
::非金属的特性包括一个相对较低的沸点,因此许多非金属是气体,非金属也是发热不良的导体,固态的非金属枯燥和易碎。 -
Some nonmetals are very reactive, whereas others are not reactive at all. It depends on the number of electrons in their outer energy level.
::有些非金属反应性很强,而另一些则完全没有反应性,这取决于其外能水平中的电子数量。 -
Reactive nonmetals tend to gain electrons. This explains why they cannot conduct electricity, which is a flow of electrons.
::反应性非金属倾向于获得电子。 这解释了为什么它们不能用电,这是电子流动。
Review
::回顾-
What are nonmetals?
::什么是非金属? -
List properties of nonmetals.
::非金属清单属性。 -
Explain why nonmetals vary in their reactivity.
::解释为什么非金属的活性不同。 -
Sulfur
cannot conduct electricity. Why not?
::硫磺不能用电 为什么不呢?
Explore More
::探索更多Watch the video about nonmetals below , and then answer the questions that follow .
::观看下面关于非金属的视频, 然后回答接下来的问题。-
The science teacher in the video does an experiment in which he tests the reactivity of four nonmetal gases. How does he test them?
::录像中的科学老师做一个实验 测试四种非金属气体的活性 他如何测试它们? -
What is the outcome of the experiment?
::实验的结果是什么? -
Based on this outcome, what conclusion(s) can you draw?
::基于这一结果,你能得出什么结论? -
Why do the gases differ in reactivity?
::为什么气体在反应上有所不同?
-
Nonmetals are elements that generally cannot conduct electricity. They are the second largest class of elements after metals. Examples of nonmetals include hydrogen, carbon, chlorine, and helium.