能源
章节大纲
-
These cheerleaders have a lot of energy! Their role is to get the fans as excited as they are so everyone will cheer for the team. They use their to activate the crowd.
::这些啦啦队员有很多精力! 他们的作用是让球迷兴奋起来,让大家为球队欢呼。他们利用球队来激活人群。Getting Started
::开始also need energy to be activated. They require a certain amount of energy just to get started. This energy is called activation energy . For example, activation energy is needed to start a car engine. Turning the key causes a spark that activates the burning of gasoline in the engine. The combustion of won’t occur without the spark of energy to begin the reaction.
::也需要激活能源。 它们需要一定数量的能源才能启动。 这种能源被称为激活能源。 例如, 启动汽车引擎需要激活能源。 将关键电源引致燃燃汽油的火花。 没有能量的火花就不会发生燃烧。Q: Why is activation energy needed? Why won’t a reaction occur without it?
::问题:为何需要激活能源? 为何没有能源就不会发生反应?A: A reaction won’t occur unless atoms or molecules of reactants come together. This happens only if the particles are moving, and movement takes energy. Often, reactants have to overcome forces that push them apart. This takes energy as well. Still more energy is needed to start breaking bonds in reactants.
::问题:除非反应器的原子或分子聚集在一起,否则反应不会发生。 只有当粒子在移动,运动需要能量时才会发生。 通常,反应器必须克服驱散它们的力量。 这也需要能量。 还需要更多能量才能打破反应器的联结。Activating Endothermic and Exothermic Reactions
::激活内热和室外热反应Some chemical reactions need a constant input of energy to take place. They are called endothermic reactions. Other chemical reactions release energy when they occur, so they can keep going without any added energy. They are called .
::一些化学反应需要不断投入能量才能发生。它们被称为内温反应。其他化学反应一旦发生就会释放能量,这样它们就可以不增加任何能量而继续前进。它们被称为 。Q: It makes sense that endothermic reactions need activation energy. But do exothermic reactions also need activation energy?
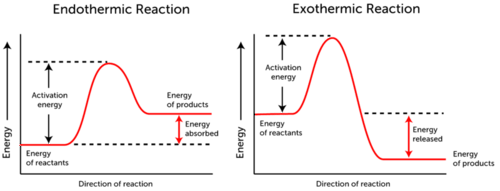
::问题:最终的热反应需要激活能量是有道理的。但异热反应也需要激活能量吗?A: All chemical reactions need energy to get started, even exothermic reactions. Look at the Figure . They compare energy changes that occur during endothermic and exothermic reactions. From the graphs, you can see that both types of reactions need the same amount of activation energy in order to get started. Only after it starts does the exothermic reaction produce more energy than it uses.
::A: 所有的化学反应都需要能量才能启动, 甚至是异温反应。 看看图。 它们比较了在异热反应和异热反应中发生的能量变化。 从图表中, 您可以看到两种反应都需要相同的激活能量才能启动。 只有开始之后, 异热反应才能产生比它使用的更多的能量。A Common Example
::共同示例You have probably used activation energy to start a chemical reaction. For example, if you’ve ever struck a match to light it, then you provided the activation energy needed to start a . When you struck the match on the box, the started the match head burning. Combustion is exothermic. Once a match starts to burn, it releases enough energy to activate the next reaction, and the next, and so on. However, the match won’t burst into flames on its own.
::您可能已经使用了激活能量来启动一种化学反应。 例如,如果你曾经打过匹配点燃它,那么你就会提供启动它所需的激活能量。 当你在盒子上打过比赛时,就会开始火柴头燃烧。 燃烧是异热的。 一旦火柴开始燃烧,它就会释放出足够的能量来激活下一个反应,而下一个反应,等等。 然而,比赛本身不会爆发火焰。Summary
::摘要-
All chemical reactions, including exothermic reactions, need activation energy to get started.
::所有化学反应,包括异温反应 都需要激活能量才能启动 -
Activation energy is needed so reactants can move together, overcome forces of repulsion, and start breaking bonds.
::需要激活能源,以便反应者能够共同移动,克服反弹力,并开始打破债券。
Review
::回顾-
What is activation energy?
::什么是激活能量? -
Why do reactants need energy in order for a chemical reaction to begin?
::为什么反应者需要能源才能开始化学反应? -
Make an original graph to represent the role of energy, including activation energy, in an endothermic reaction.
::用原始图表表示能量的作用, 包括激活能量, 在局部热反应中的作用。
-
All chemical reactions, including exothermic reactions, need activation energy to get started.