Beta 衰变
章节大纲
-
If you hear the word decay while your dentist is checking your teeth, it’s probably bad news. But if you’re an unstable atomic nucleus , decay is good news. When the nucleus of an decays, it becomes more stable.
::如果你在牙医检查牙齿时听到“腐烂”这个词,那很可能是坏消息。 但如果你是一个不稳定的原子核,腐烂就是好消息。 当腐烂的核心变得更加稳定时,它就会变得更加稳定。Which Nuclei Decay?
::哪个纽克莱·德凯?Atoms with unstable nuclei are radioactive. To become more stable, the nuclei undergo . In radioactive decay, the nuclei emit and usually particles of matter as well. There are several , including alpha, beta, and . Energy is emitted in all three types of decay, but only alpha and beta decay also emit particles.
::具有不稳定核的原子是放射性的。要变得更稳定,核将经历。在放射性衰变中,核会释放,通常物质粒子也会释放。有几种物质,包括α、β和。能量是在所有三种类型的衰变中释放的,但只有α和β的衰变也会释放粒子。What Is Beta Decay?
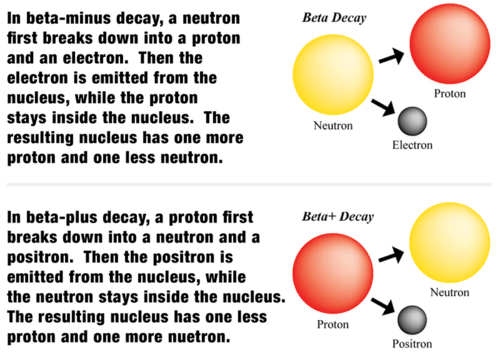
::什么是贝塔衰变?Beta decay occurs when an unstable nucleus emits a beta particle and energy. A beta particle is either an or a positron. An electron is a negatively charged particle, and a positron is a positively charged electron (or anti-electron). When the beta particle is an electron, the decay is called beta-minus decay. When the beta particle is a positron, the decay is called beta-plus decay. Beta-minus decay occurs when a nucleus has too many relative to , and beta-plus decay occurs when a nucleus has too few neutrons relative to protons.
::当不稳定核释放贝塔粒子和能量时,Beta 衰变就会发生。贝塔粒子要么是正数,要么是正数。电子是负电荷粒子,而正数是正电荷电子(或反电子)。当贝塔粒子是电子时,衰变就被称为乙-minus衰变。当贝塔粒子是正数时,衰变就被称为β-plus 衰变。当核与质子相比数量过多时,Beta-minus 衰变就会发生,当核与质相比的中子太少时,则会发生乙-plus衰变。Q: Nuclei contain only protons and neutrons, so how can a nucleus emit an electron in beta-minus decay or a positron in beta-plus decay?
::问题:核仅含有质子和中子,所以核如何在β-minus衰变中排放电子,或乙-plus衰变中释放阳子?A: Beta decay begins with a proton or neutron. You can see how in the Figure .
::A:Beta衰变始于质子或中子。您可以从图中看到。Q: How does beta decay change an atom to a different ?
::问题:贝塔衰变如何将原子改变为不同?A: In beta-minus decay an atom gains a proton, and in beta-plus decay it loses a proton. In each case, the atom becomes a different element because it has a different number of protons.
::A:在β-minus衰减时,原子会增加质子,而在β-+衰减时,原子会失去质子。 在每一种情况下,原子会因质子数量不同而成为不同的元素。Equations for Beta Decay
::Beta 衰变的等值Radioactive nuclei and particles are represented by nuclear symbols.. For example, a beta-minus particle (electron) is represented by the symbol . The subscript -1 represents the particle’s charge, and the superscript 0 shows that the particle has virtually no mass (no protons or neutrons). Another example is the radioactive nucleus of thorium-234. It is represented by the symbol , where the subscript 90 stands for the number of protons and the superscript 234 for the number of protons plus neutrons.
::放射性核和粒子由核符号代表。例如,β-minus粒子(电子)由符号 - 10e代表。下标-1代表粒子的充电,上标0显示粒子几乎没有质量(没有质子或中子 ) 。另一个例子是-234的放射性核。它由符号 90234TH代表,下标90代表质子的数量,上标234代表质子和中子的数量。Nuclear symbols are used to write nuclear equations for radioactive decay. Let’s consider the example of the beta-minus decay of thorium-234 to protactinium-234. This reaction is represented by the equation:
::核符号被用来为放射性衰变写核方程式。 让我们来看看二三四的乙基-毫微衰变到丙二-234的例子。 这种反应表现为方程式:-
-
- → + + energy
-
The equation shows that thorium-234 becomes protactinium-234 and loses a beta particle and energy. The protactinium-234 produced in the reaction is also radioactive, so it will decay as well.
::方程式显示234变成234,并失去乙型粒子和能量。反应中产生的234也是放射性的,因此也会腐烂。A nuclear equation is balanced if the total numbers of protons and neutrons are the same on both sides of the arrow. If you compare the subscripts and superscripts on both sides of the equation above, you’ll see that they are the same.
::如果箭头两侧质子和中子的总数相同,则核方程式是平衡的。 如果你比较上面方程式两侧的下标和上标, 你会看到它们是一样的。Q: What happens to the electron produced in the reaction above?
::问题:上述反应中产生的电子会怎么样?A: Along with another electron, it can combine with an alpha particle to form a helium atom. An alpha particle, which is emitted during , consists of two protons and two neutrons.
::A:与另一种电子一起,它可以与一个阿尔法粒子结合形成一个原子。一个阿尔法粒子,由两个质子和两个中子组成,该粒子在 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Q: Try to balance the following nuclear equation for beta-minus decay by filling in the missing subscript and superscript.
::问题:试图通过填充缺失的下标和上标来平衡β-负衰变的以下核方程。-
-
- → + + energy
-
A: The subscript of Xe is 54, and the superscript is 131.
::A:Xe的下标为54,上标为131。Dangers of Beta Decay
::贝塔衰变的危险Beta particles can travel about a meter through air. They can pass through a sheet of paper or a layer of cloth but not through a sheet of aluminum or a few centimeters of wood. They can also penetrate the skin and damage underlying tissues. They are even more harmful if they are ingested or inhaled.
::贝塔粒子可以在空气中穿行约一米。它们可以通过纸板或一层布,但不能通过铝板或几厘米的木头。它们也可以穿透皮肤,损害底部组织。如果吸食或吸入,它们更有害。Summary
::摘要-
Beta decay occurs when a nucleus is unstable because it has too many or too few neutrons relative to protons. The nucleus emits a beta particle and energy. A beta particle is either an electron (beta-minus decay) or a positron (beta-plus decay).
::当核因与质子相比的中子过多或太少而不稳定时,Beta会发生衰变。核会释放贝塔粒子和能量。贝塔粒子要么是电子(贝塔-最小衰变),要么是正电子(贝塔-倍增衰变)。 -
In beta-minus decay, a neutron breaks down to a proton and an electron, and the electron is emitted from the nucleus. In beta-plus decay, a proton breaks down to a neutron and a positron, and the positron is emitted from the nucleus.
::在β-minus衰变中,中子分解成质子和电子,电子从核中释放出来。在β-plus衰变中,质子分解成中子和阳子,而正子则从核中释放出来。 -
Balanced nuclear equations show how the numbers of protons and neutrons change in beta decay.
::平衡的核方程式表明 质子和中子的数量 是如何改变贝塔衰变的 -
Beta radiation is harmful to living things.
::贝塔辐射对生物有害
Review
::回顾-
Compare and contrast beta-minus and beta-plus decay.
::对比和对比β-minus 和β-plus 衰变。
-
Fill in the missing subscript and superscript in this nuclear equation to balance it:
::填充核方程式中缺失的下标和上标 以平衡它:
→ + + energy
::+ -10e+能源Does the equation represent beta-minus or beta-plus decay? How do you know?
::等式是否代表 β- minus 或 β- plus 衰减 ? 你怎么知道 ?Resources
::资源 -