半衰期
Section outline
-
Assume that you cut a sheet of paper down the center to get two halves. Then you cut each half down the center to get four pieces. If you keep cutting the pieces of paper in half, you would soon a reach a point where the pieces are too small to cut again. A radioactive is a little like that sheet of paper.
::假设您在中间切下一张纸, 以获得两半。 然后您将每半切下中间, 以获得四块。 如果您继续切成两半, 您很快会到达一个点, 碎片太小, 无法再切。 放射性像一张纸一样。What Is a Radioactive Isotope?
::什么是放射性同位素?A radioactive isotope, or , has atoms with unstable nuclei . The unstable nuclei naturally decay, or break down, by losing and particles of matter to become more stable. If they gain or lose as they decay, they become different . Over time, as the nuclei continue to decay, less and less of the original radioisotope remains.
::放射性同位素(或 ) 具有不稳定核的原子。 不稳定核的自然自然衰减,或通过流失而分解,物质粒子变得更加稳定。 如果他们随着衰变而增减,它们就会变得不同。 随着核的继续衰减,原放射性同位素的残存越来越少。Rate of Radioactive Decay
::放射性衰减率A radioisotope decays and changes to a different element at a constant rate. The rate is measured in a unit called the half-life . This is the length of time it takes for half of a given amount of the radioisotope to decay. This rate is always the same for a given radioisotope, regardless of , pressure , or other conditions outside the nuclei of its atoms.
::放射性同位素以恒定速率衰减和改变一个不同的元素。该速率用一个称为半衰期的单位测量。这是放射性同位素衰减一半所需的时间长度。对于特定放射性同位素而言,这一速率总是相同的,不管其原子核外有何种压力或其他条件。Q: How is repeatedly cutting paper in half like the decay of a radioisotope?
::问:像放射性同位素衰变一样 不断切纸一半是怎么样的?A: As a radioisotope decays, the amount of the radioisotope decreases by half during each half-life, just as a piece of paper decreases in size by half each time you cut it down the center.
::A:随着放射性同位素衰减,放射性同位素的数量在每个半衰期中减少一半,就像一块纸在每次剪切中心时减少一半一样。Half-Life Example
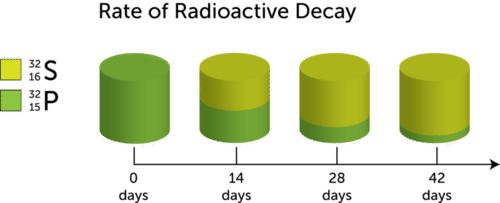
::半衰期示例The concept of half-life is illustrated in the Figure for the decay of phosphorus-32 to sulfur-32. The half-life of phosphorus-32 is 14 days. After 14 days, half of the original amount of phosphorus-32 has decayed, so only half remains. After another 14 days, half of the remaining amount (or a quarter of the original amount) is still left, and so on.
::半衰期概念在磷-32到硫-32的衰减图中作了说明。磷-32的半衰期为14天。14天后,原磷-32的一半已经衰减,因此只剩下一半。再过14天后,剩余的一半(或原量的四分之一)仍然剩下,等等。Q: What fraction of the original amount of phosphorus-32 remains after three half-lives?
::问题:原磷-32含量的哪些部分在三分半衰期之后还剩下?A: After three half-lives, or 42 days, 1/8 (1/2 × 1/2 × 1/2) of the original amount of phosphorus-32 remains.
::A:经过3半衰期或42天后,原磷-32含量的1/8(1/2×1/2×1/2×1/2)残余物。Variation in Half-Lives
::半生命的变异Different radioisotopes may vary greatly in their rate of decay. That’s because they vary in how unstable their nuclei are. The more unstable the nuclei, the faster they break down. As you can see from the examples in the Table , the half-life of a radioisotope can be as short as a split second or as long as several billion years.
::不同的放射性同位素在衰减率上可能差异很大。 这是因为它们的核心不稳定程度不同。 核心越不稳定,其分解速度就越快。 从表中的例子中可以看出,放射性同位素的半衰期可能短于二分之一或长达数十亿年。Isotope Half-life Uranium-238 4.47 billion years Potassium-40 1.28 billion years Carbon-14 5,700 years Hydrogen-3 12.3 years Radon-222 3.82 days Polonium-214 0.00016 seconds Q: If you had 1 gram of carbon-14, how many years would it take for to reduce it to 1/4 gram?
::问:如果你有1克碳14, 需要多少年才能减少到1/4克?A: 1 gram would decay to ¼ gram in 2 half-lives. One half-life is 5,700 years, so two half-lives are 11,400 years.
::A:1克半衰期将衰减到1/4克,半衰期为5,700年,半衰期为11,400年。Summary
::摘要-
A radioisotope decays and changes to a different element at a certain constant rate called the half-life. This is the length of time it takes for half of a given amount of the radioisotope to decay.
::放射性同位素衰变和以某种固定速度改变一个不同的元素,称为半衰期。这就是放射性同位素衰变所需时间的长度,是给定数量的一半放射性同位素衰变所需的时间。 -
Different radioisotopes may vary greatly in their rate of decay. The more unstable their nuclei are, the faster they decay.
::不同放射性同位素的衰变速度可能大不相同。它们的核越不稳定,其衰变速度就越快。
Review
::回顾-
Define half-life.
::界定半衰期。 -
Why do radioisotopes differ in the length of their half-lives?
::为什么放射性同位素的半衰期长度不同? -
What fraction of a given amount of hydrogen-3 would be left after 36.9 years of decay? (
Hint
: Find the half-life of hydrogen-3 in the
Table
.)
::在36.9年的衰变后,一定数量的氢-3将留下多少部分? (提示:在表格中查找氢-3的半衰期。 )
-
A radioisotope decays and changes to a different element at a certain constant rate called the half-life. This is the length of time it takes for half of a given amount of the radioisotope to decay.