3.1原子
Section outline
-
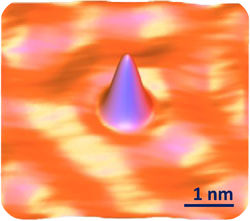
What could this hilly blue surface possibly be?
::这个山地蓝色的表面会是什么?Do you have any idea? The answer is a single atom of the cobalt. The picture was created using a scanning tunneling microscope. No other microscope can make images of things as tiny as atoms. How small are atoms? You will find out in this lesson.Discovering the Atom
::发现原子Atoms are very tiny, so tiny that they could not be seen before scanning tunneling microscopes were invented in 1981. However, the atom and its parts were discovered long before the tunneling microscope was invented. How did scientists discover the atom if they couldn’t see it? They made models. A scientific model is a tool constructed by the scientist based on all the known experimental evidence about a particular thing, such as an atom. The first model of the atom goes back to ancient Greece. As time went by and more are performed, models evolve and change to account for new understanding. Explore the timeline below to see how the model of the atom was initially developed and how it has changed over time into what we now have come to accept as the modern model of the atom.Atoms
::原子原子Atoms are the building blocks of matter. They are the smallest particles of an element that still have the element's properties. Elements, in turn, are pure substances—such as nickel, hydrogen, and helium—that make up all kinds of matter. All the atoms of a given element are identical in that they have the same number of , one of the building blocks of atoms (see below). They are also different from the atoms of all other elements, as atoms of different elements have different numbers of protons.Size of Atoms
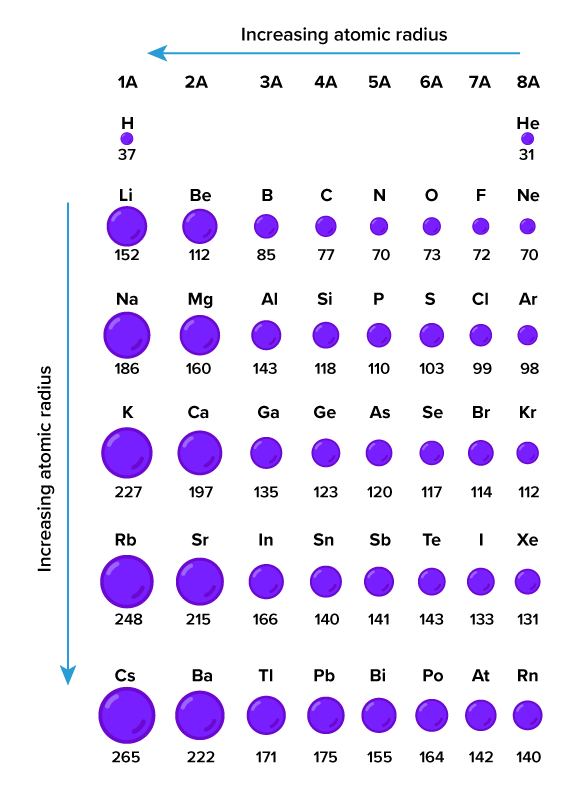
::原子的大小Unlike bricks, atoms are extremely small. The radius of an atom is well under 1 nanometer, which is one billionth of a meter. If a size that small is hard to imagine, consider this: trillions of atoms would fit inside the period at the end of this sentence. Although all atoms are very small, elements vary in the size of their atoms. The Figure compares the sizes of atoms of more than 40 different elements, showing their atomic radii in picometers (pm), which is the most common unit used to measure atomic radius. The elements in the figure are represented by chemical symbols, such as H for hydrogen and He for helium. Of course, real atoms are much smaller than the circles representing them in the Figure below.Atomic size chart
::原子面积图The atomic radii of many elements are shown above. Atomic Radii are measured in picometers (pm). Q: Which element in the chart above has the biggest atoms?
::问题:上图中哪个元素有最大的原子?A: According to the chart above, the element with the biggest atoms is cesium (Cs).
::甲:根据上图,最大原子的元素是(Cs)。Subatomic Particles
::亚原子粒子Although atoms are very tiny, they consist of even smaller particles. Three main types of particles that make up all atoms are protons, , and electrons. The interactive below shows how these particles are arranged in an atom. Click on the subatomic particle to learn more about each type.The atom shown above represented by the model is carbon , but the particles of all atoms are arranged in the same way. At the center of the atom is a dense area called the nucleus , where all the protons and neutrons are clustered closely together. The electrons constantly move around the nucleus. Helium has two protons and two neutrons in its nucleus and two electrons moving around the nucleus. Atoms of other elements have different numbers of subatomic particles, but the number of protons always equals the number of electrons. This makes atoms neutral in charge because the positive and negative charges "cancel out."Q: Helium has two protons, two neutrons, and two electrons. Sketch a model of a helium atom, similar to the model above for carbon.A: Does your sketch resemble the model in the image below?
::A:您的素描与下面图像中的模型相似吗?Q: All atoms of carbon have six protons. How many electrons do carbon atoms have?
::问题:所有原子的碳有6个质子。碳原子有多少个电子?A: Carbon atoms must have six electrons to "cancel out" the positive charges of the six protons. Atoms are always neutral in charge, containing the same number of electrons as protons. Losing or gaining electrons from this balance converts them into .
::A: 碳原子必须有六种电子来“ 取消” 六个质子的正电荷。 原子始终是中性负责的, 其电子数量与质子相同。 从此平衡中丢失或获取电子可以将其转换为 。Summary
::摘要-
Atoms are the building blocks of matter.
::原子是物质的构件 -
They are the smallest particles of an element that still have the element's properties. All atoms are very small, but atoms of different elements vary in size.
::它们是一个元素的最小颗粒, 仍然具有元素的特性。 所有原子都非常小, 但不同元素的原子大小不同 。 -
Three main types of particles that make up all atoms are protons, neutrons, and electrons.
::构成所有原子的三种主要颗粒类型是质子、中子和电子。
Review
::回顾-
What is an atom?
::什么是原子? 原子吗? -
Which of the following statement(s) are true about the atoms of any element?
-
The number of protons in an atom of an element is unique to each element.
::元素原子中的质子数是每个元素独有的。 -
The number of protons and neutrons in an atom of an element is unique to each element.
::元素原子中的质子和中子数量是每个元素独有的。 -
A proton is an atom of one element that is identical to a proton in an atom of another element.
::质子是指一个元素的原子,与另一个元素的原子中的质子相同。 -
The number of protons in an atom of an element is the same for all elements.
::元素原子中的质子数量对所有元素都是相同的。
::下列哪些语句对任何元素的原子是真实的? 元素原子中的质子数量是每个元素独有的。 元素原子中的质子和中子数量是每个元素独有的。 质子是一个元素原子的原子, 与另一个元素的原子中的质子相同。 元素原子中的质子数量对所有元素都是相同的。 -
The number of protons in an atom of an element is unique to each element.
-
Which of the following statements explains why atoms are always neutral in charge
-
They have the same number of protons as the atoms of all other elements.
::它们的质子数量与所有其他元素的原子数量相同。 -
They have protons that are identical to the protons of all other elements.
::它们拥有与所有其他元素的质子相同的质子。 -
They have the same size as the atoms of all other elements.
::它们与所有其他元素的原子大小相同。 -
They have the same number of protons as electrons.
::它们的质子数量与电子数量相同
::以下哪些语句解释了为什么原子总是中性负责的原因,它们拥有与所有其他元素原子相同的质子数量。它们拥有与所有其他元素质子相同的质子数量。它们拥有与所有其他元素质子相同的质子。它们拥有与所有其他元素原子相同的大小。它们拥有与电子相同的质子数量。 -
They have the same number of protons as the atoms of all other elements.
-
Atoms are the building blocks of matter.