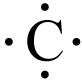4.6 瓦伦斯电
Section outline
-
Did you ever play the card game called "Go fish"? Players try to form groups of cards of the same value, such as four sevens, with the cards they are dealt or by getting cards from other players or the deck. This give and take of cards is a simple analogy for the way atoms give and take valence electrons in .
::玩牌游戏时玩过“ 去钓鱼 ” 吗 ? 玩家们试图将牌类组合成相同价值的牌类, 比如四七张牌, 牌牌由他们处理, 或者从其他玩家或甲板上获取牌。 这对原子给付和接收值电子的方式来说, 给付和拿取牌是一个简单的类比 。What Are Valence Electrons?
::什么是瓦伦斯电子?Valence electrons are the electrons in the outer of an that can participate in interactions with other atoms. Valence electrons are generally the electrons that are farthest from the nucleus . As a result, they may be attracted as much or more by the nucleus of another atom than they are by their own nucleus.
::Valence 电子是能够参与与其他原子相互作用的电子的外部电子。 Valence 电子通常是距离核最远的电子。 结果,它们被另一个原子的核心吸引的程度可能比它们自己的核心要大或多。Electron Dot Diagrams
::电子点图图Because valence electrons are so important, atoms are often represented by simple diagrams that show only their valence electrons. These are called dot diagrams, and three are shown below. In this type of diagram, an element's chemical symbol is surrounded by dots that represent the valence electrons. Typically, the dots are drawn as if there is a square surrounding the symbol with up to two dots per side. An element never has more than eight valence electrons, so there can’t be more than eight dots per atom.
::由于价值电子如此重要,原子通常由简单的图表来代表,这些图表只显示其价值电子。这些图表被称为点图,下面显示三个。在这类图表中,元素的化学符号被代表价值电子的点所包围。通常,点像符号周围有一个方形,每侧最多有两个点。一个元素从未超过8个值电子,因此每个原子的值电子不超过8个点。Q: Carbon (C) has four valence electrons. What does an electron dot diagram for this element look like?
::Q:碳(C)有四个值电子。电子点图对这个元素的外观是什么?A: An electron dot diagram for carbon looks like this:
::A:一个碳电子点图是这样的:Valence Electrons and the Periodic Table
::电压电和周期表The number of valence electrons in an atom is reflected by its position in the periodic table of the elements (see the periodic table in the Figure ). Across each row, or period, of the periodic table, the number of valence electrons in groups 1–2 and 13–18 increases by one from one element to the next. Within each column, or group, of the table, all the elements have the same number of valence electrons. This explains why all the elements in the same group have very similar chemical properties.
::原子中值电子的数量反映在元素周期表中的位置(见图中的周期表 ) 。 在周期表的每行或周期中,1-2组和13-18组的值电子数量从一个元素到下一个元素增加一个元素。在表格的每列或每组中,所有元素的值电子数量都相同。这解释了为什么同一组的所有元素都具有非常相似的化学特性。For elements in groups 1–2 and 13–18, the number of valence electrons is easy to tell directly from the periodic table. This is illustrated in the simplified periodic table in the Figure . It shows just the numbers of valence electrons in each of these groups. For elements in groups 3–12, determining the number of valence electrons is more complicated.
::对于第1-2组和第13-18组中的元素,价值电子的数量很容易从周期表直接看出。图中的简化周期表说明了这一点。它只显示了这些组中每一组中价值电子的数量。对于第3-12组中的元素,确定价值电子的数量更为复杂。Q: Based on both periodic tables above ( Figures and ), what are examples of elements that have just one valence electron? What are examples of elements that have eight valence electrons? How many valence electrons does oxygen (O) have?
::问题:基于以上两个周期表(图和),仅有一个值电子的元素有哪些示例?有八个值电子的元素有哪些示例?氧(O)有多少值电子?A: Any element in group 1 has just one valence electron. Examples include hydrogen (H), lithium (Li), and sodium (Na). Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include neon (Ne), argon (Ar), and krypton (Kr). Oxygen, like all the other elements in group 16, has six valence electrons.
::A组:第1组中的任何元素只有一个valence 电子,例如氢(H)、锂(Li)和钠(Na)。第18组中的任何元素都有八种valence 电子(但除外,它总共只有两个电子)。例子包括纳米(Ne)、argon(Ar)和Krypton(Kr)。Oxygen与第16组中的所有其他元素一样,有六种valene。Valence Electrons and Reactivity
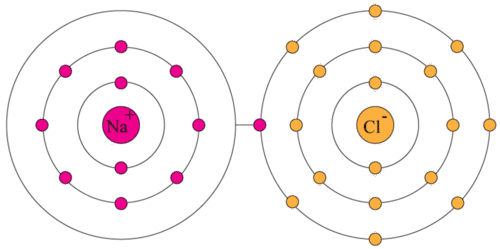
::VALE 电和反应电The table salt pictured in the Figure contains two elements that are so reactive they are rarely found alone in nature. Instead, they undergo chemical reactions with other elements and form compounds. Table salt is the named sodium chloride (NaCl). It forms when an atom of sodium (Na) gives up an electron and an atom of chlorine (Cl) accepts it. When this happens, sodium becomes a positively charged (Na + ), and chlorine becomes a negatively charged ion (Cl - ). The two ions are attracted to each and join a matrix of interlocking sodium and chloride ions, forming a crystal of salt.
::图中绘制的表盐含有两个反应性极强的元素,这些元素在性质上很少被单独发现,而是与其他元素和形式化合物发生化学反应。表盐称为氯化钠(NaCl)。当一个原子(Na)放弃一个电子,一个原子(Cl)接受一个氯(Cl)时,就会形成一个原子。当发生这种情况时,钠会变成正电(Na+),而氯会变成一个负电离子(Cl-)。两种离子被吸引到其中,并加入一个将钠和氯化离子连接在一起的矩阵,形成一个盐晶体。Table salt (sodium chloride).
::食盐(氯化钠)。Q: Why does sodium give up an electron?
::问题:为什么钠会放弃电子?A: An atom of a group 1 element such as sodium has just one valence electron. It is “eager” to give up this electron in order to have a full outer energy level, because this will give it the most stable arrangement of electrons. You can see how this happens in the Figure . Group 2 elements with two valence electrons are almost as reactive as elements in group 1 for the same reason.
::A: 钠等第1组元素的原子只有一个valence 电子。 它“ 渴望” 放弃该电子, 以便拥有一个完整的外部能量水平, 因为这将使它拥有最稳定的电子配置。 您可以在图中看到这种情况是如何发生的。 具有两个valence 电子的第2组元素与第1组元素一样具有反应性, 出于同样的原因。Q: Why does chlorine accept the electron from sodium?
::问题:为什么氯接受钠的电子?A: An atom of a group 17 element such as chlorine has seven valence electrons. It is “eager” to gain an extra electron to fill its outer energy level and gain stability. Group 16 elements with six valence electrons are almost as reactive for the same reason.
::A:氯等17组元素的原子有7种价值电子,“渴望”获得额外电子以填充其外部能量水平并获得稳定性。 16组元素,包括6种价值电子,由于同样的原因,几乎与反应一样。Atoms of group 18 elements have eight valence electrons (or two in the case of helium). These elements already have a full outer energy level, so they are very stable. As a result, they rarely if ever react with other elements. Elements in other groups vary in their reactivity but are generally less reactive than elements in groups 1, 2, 16, or 17.
::第18组元素的原子有8种等值电子(在氦的情况下有2种),这些元素已经具有完全的外向能量水平,因此非常稳定。因此,它们很少与其他元素发生反应。其他组的元素在反应性方面各不相同,但一般比第1、2、16或17组的元素反应较少。Q: Find calcium (Ca) in the periodic table (see Figure ). Based on its position in the table, how reactive do you think calcium is? Name another element with which calcium might react.
::Q: 在周期表(见图 ) 中查找钙 (Ca) 。根据其在表格中的方位,您认为钙是如何反应的? 列出钙可能反应的另一个元素 。A: Calcium is a group 2 element with two valence electrons. Therefore, it is very reactive and gives up electrons in chemical reactions. It is likely to react with an element with six valence electrons that “wants” to gain two electrons. This would be an element in group 6, such as oxygen.
::A: 钙是第2组元素,有两个值电子。 因此, 它非常反应性, 在化学反应中放弃电子。 它可能与六个值电子发生反应, 以“ wants” 获得两个电子。 这将是第6组中的一个元素, 比如氧气 。Valence Electrons and Electricity
::电电电Valence electrons also determine how well—if at all—the atoms of an element conduct electricity. The copper wires in the cable in the Figure are coated with plastic. Copper is an excellent conductor of electricity, so it is used for wires that carry electric current . Plastic contains mainly carbon, which cannot conduct electricity, so it is used as insulation on the wires.
::瓦伦斯电子还确定一个元素的原子能进行电动的程度(如果有的话)如何。图中电缆中的铜线涂有塑料。铜是极好的电力导电器,因此用于携带电流的电线。塑料主要是碳,不能进行电,因此用作电线上的绝缘。Q: Why do copper and carbon differ in their ability to conduct electricity?
::问题:为什么铜和碳的发电能力不同?A: Atoms of such as copper easily give up valence electrons. Their electrons can move freely and carry electric current. Atoms of such as the carbon, on the other hand, hold onto their electrons. Their electrons can’t move freely and carry current.
::A:铜等原子很容易放弃价值电子。 它们的电子可以自由移动并携带电流。 而碳等原子则紧握在自己的电子上。 它们的电子不能自由移动并携带电流。A few elements, called , can conduct electricity, but not as well as metals. Examples include silicon and germanium in group 14. Both become better conductors at higher temperatures. These elements are called semiconductors.
::被称为 " 几个元素 " 的一些元素可以进行电力,但不能像金属一样进行电力,例如14组中的硅和。这两种元素在较高温度下都成为更好的导导体。这些元素被称为半导体。Q: How many valence electrons do atoms of silicon and germanium have? What happens to their valence electrons when the atoms are exposed to an electric field ?
::问题:硅和原子有多少价值电子?当原子接触电场时,其价值电子会怎样?A: Atoms of these two elements have four valence electrons. When the atoms are exposed to an electric field, the valence electrons move away from the atoms and allow current to flow.
::A:这两个元素的原子有四个值电子。当原子接触电场时,值电子从原子移开,让水流流动。Summary
::摘要-
Valence electrons are the electrons in the outer energy level of an atom that can participate in interactions with other atoms.
::等离子电子是原子外能层中的电子,可以参与与其他原子的相互作用。 -
Electron dot diagrams that show only the valence electrons present in an atom.
::电子点图只显示原子中存在的值电子。 -
The number of valence electrons in atoms may cause them to be unreactive or highly reactive. For those atoms that are reactive, the number of valence electrons also determines whether they tend to give up or gain electrons in chemical reactions.
::原子中的等值电子数量可能导致它们不反应或高度反应。 对于反应中的原子来说,等值电子数量也决定它们是否倾向于放弃或获取化学反应中的电子。 -
Metals, which easily give up electrons, can conduct electricity. Nonmetals, which attract electrons, generally cannot. Metalloids such as silicon and germanium can conduct electricity but not as well as metals.
::金属很容易放弃电子,可以进行电力生产。 吸引电子的非金属通常无法进行。 硅和等类金属可以进行电力生产,但不能进行金属生产。
Review
::回顾-
What are valence electrons?
::什么是价值电子? -
Draw an electron dot diagram for an atom of nitrogen (N).
::为氮原子绘制电子点图( N) 。 -
Which of the following statements about valence electrons and the periodic table is true?
-
The number of valence electrons decreases from left to right across each period.
::每段期间,保值电子从左向右下降。 -
The number of valence electrons increases from top to bottom within each group.
::每个组内价值电子从上到下增加的数量。 -
All of the elements in group 9 have nine valence electrons.
::第9组的所有元素都有九种值电子 -
Elements with the most valence electrons are in group 18.
::价值最高的电子元素在18组。
::有关值值电子和周期表的以下哪些语句是真实的? 值电子的数量在每个周期从左向右下降。 值电子的数量在每个组内从上向下增加。 第9组的所有元素都有九种值电子。 价值电子中最强的元素在第18组。 -
The number of valence electrons decreases from left to right across each period.
-
Which element would you expect to be more reactive: phosphorus (P) or fluorine (F)? Explain your answer.
::您希望哪个元素更具反应性:磷(P)或氟(F)? 请解释您的答复。 -
Why can’t nonmetals conduct electricity?
::为何非金属不能用电?
-
Valence electrons are the electrons in the outer energy level of an atom that can participate in interactions with other atoms.