6.3 晶碳形式
Section outline
-
How annoying! Just as you start to write in your notebook, your pencil lead breaks. Actually, pencil “leads” aren’t made of lead at all. They are made of a form of carbon called graphite. How carbon atoms are arranged in graphite explains why it’s suitable for writing—and also why it breaks so easily.
::多么令人烦恼! 就像你开始在笔记本上写笔铅笔的破折。 事实上,铅笔“铅笔”根本不是铅做的。 铅笔“铅笔”是由碳石墨组成的。 石墨中如何安排碳原子解释了为什么它适合写作 — — 也解释了为什么它很容易破解。Q: Graphite is one of several forms of carbon. How can a single exist in different forms?
::问题:石墨是多种碳形式之一。 单一碳如何以不同形式存在?A: Carbon can exist in different forms because atoms of carbon can combine in different ways.
::A:碳可以不同形式存在,因为碳原子可以不同方式结合。What Are the Forms of Carbon?
::什么是碳形式?Graphite is one of three forms of crystalline, or crystal-forming, carbon. Carbon also exists in an amorphous, or “shapeless,” form in substances such as coal and charcoal. Different forms of the same element are called allotropes. Besides graphite, the other allotropes of crystalline carbon are diamond and fullerenes. All three forms exist as crystals rather than molecules. In a crystal , many atoms are bonded together in a repeating pattern that may contains thousands of atoms. The arrangement of atoms in the crystal differs for each form of carbon and explains why the different forms have different properties.
::石墨是三种晶状或晶体成形碳的形式之一。碳也存在于煤炭和木炭等物质中,一种无定形或“无形”的形式。同一元素的不同形式被称为异质。除石墨外,其他晶状碳的同源体是钻石和更白的。所有三种形式都以晶体而不是分子的形式存在。在晶体中,许多原子以重复的形态结合在一起,其中可能含有数千原子。晶体中的原子的排列对每种碳形式都有不同之处,并解释了不同形式具有不同特性的原因。Q: How do you think the properties of diamond might differ from the properties of graphite?
::问题:你认为钻石的特性与石墨的特性有何不同?A: Diamond is clear whereas graphite is black. Diamond is also very hard, so it doesn’t break easily. Graphite, in contrast, is soft and breaks very easily.
::A:钻石是明确的,石墨是黑色的。 钻石也是非常困难的,因此很难打破。 相反,石墨是软的,很容易打破。Diamond
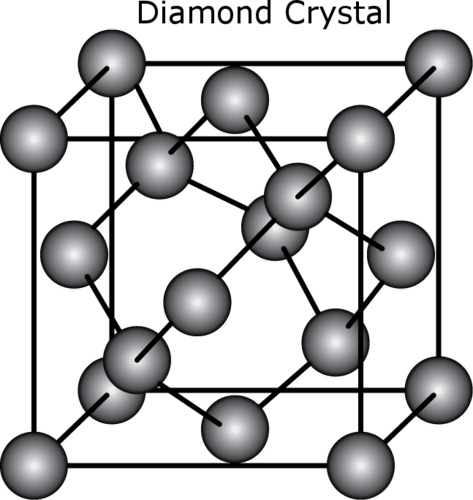
::钻石钻石钻石Diamond is a form of carbon in which each carbon is covalently bonded to four other carbon atoms. This forms a strong, rigid, three-dimensional structure (see Figure ). Diamond is the hardest natural substance, and no other natural substance can scratch it. This property makes diamonds useful for cutting and grinding tools as well as for rings and other jewelry (see Figure ).
::钻石是一种碳形式,其中每颗碳与另外四个碳原子交织在一起,形成一个坚固、僵硬、三维的结构(见图 ) 。 钻石是最硬的自然物质,没有其他自然物质可以刮去它。 这种特性使钻石用于切割和研磨工具以及环和其他首饰(见图 )。Graphite
::石墨石墨Graphite is a form of crystalline carbon in which each carbon atom is covalently bonded to three other carbon atoms. The carbon atoms are arranged in layers, with strong bonds within each layer but only weak bonds between layers (see Figure ). The weak bonds between layers allow the layers to slide over one another, so graphite is relatively soft and slippery. This makes it useful as a lubricant.
::石墨是一种晶状碳,其中每个碳原子都与另外三个碳原子相连接。碳原子按层排列,每个层内都有坚固的粘合物,但各层之间只有微弱的粘合物(见图 ) 。 层之间的微弱粘合物使层层相互滑动,因此石墨相对柔软和滑滑。 这使得它作为润滑剂有用。Q: Why do graphite’s properties make it useful for pencil “leads”?
::问题:为什么石墨的特性能让铅笔“铅笔”有用?A: Being slippery, graphite slides easily over paper when you write. Being soft, it rubs off on the paper, allowing you to leave marks. Graphite’s softness also allows you to sharpen it easily. (Imagine trying to sharpen a diamond!)
::A:滑滑,石墨在写作时很容易滑过纸面。 它柔软,在纸上擦去,允许你留下痕迹。 石墨的柔软性也使你很容易磨亮它。 (想象力试图磨亮钻石! )Fullerene
::富丽A fullerene (also called a Bucky ball) is a form of carbon in which carbon atoms are arranged in a hollow sphere resembling a soccer ball (see Figure ). Each sphere contains 60 carbon atoms, and each carbon atom is bonded to three others by two single and one double covalent bond. The bonds are relatively weak, so fullerenes can dissolve and form solutions. Fullerenes were first discovered in 1985 and have been found in soot and meteorites. Possible commercial uses of fullerenes are under investigation.
::一种更充分尼(也称为巴基球)是一种碳形式,其中碳原子排列在一个空空球中,类似于足球球(见图 ) 。 每个球体含有60个碳原子,每个碳原子与其他3个碳原子连接在一起,由两个单倍和两个双倍共价结合。 债券相对薄弱, 因此更充分尼可以溶解并形成解决方案。 富尔尼于1985年首次发现, 并被发现在烟尘和陨石中。 正在调查更美的元素的商业用途 。Fullerene Crystal Summary
::摘要-
Different forms, or allotropes, of carbon are diamond, graphite, and fullerenes.
::不同形式的碳或碳的蛋白状物是钻石、石墨和更美的元素。 -
In diamond, each carbon atom is bonded to four other carbon atoms, forming a rigid structure that makes diamond very hard.
::在钻石中,每个碳原子与其他四个碳原子相连,形成一个硬性结构,使钻石变得非常硬。 -
In graphite, each carbon atom is bonded to three other carbon atoms, and the atoms forms layers that are only weakly bonded together. This makes graphite soft and slippery.
::在石墨中,每个碳原子都结为另外三个碳原子,而原子构成的层层相互连接薄弱。这使得石墨柔软和滑滑。 -
In a fullerene, carbon atoms are bonded to three other atoms in a soccer ball pattern. The bonds are weak, so fullerenes can dissolve and form solutions.
::在一个更宽的距离中,碳原子以足球模式与其他三个原子捆绑在一起。 联系很弱,因此更宽的原子可以溶解并形成解决方案。
Review
::回顾-
Describe the bonds between carbon atoms in diamond.
::描述钻石碳原子之间的联系。 -
How does the arrangement of atoms in diamond and graphite affect their properties?
::钻石和石墨中的原子安排如何影响其特性? -
What substance is represented by the chemical formula C
60
?
::化学公式C60代表何种物质?
Explore More
::探索更多Watch the video about forms of crystalline carbon. Then answer the questions below.
::观看关于晶体碳形式的视频。 然后回答下面的问题。-
What are crystals? What are the crystalline forms of carbon?
::什么是晶体?什么是碳的晶体形式? -
Describe the unit cell of a diamond crystal.
::描述钻石晶体的单位细胞。 -
Relate pencil marks on paper to the structure of graphite.
::纸上的铅笔标记与石墨结构相对应。 -
In a Buckyball, what repeating shapes do carbon atoms form?
::在一个巴克利球里,碳原子会形成什么样的重复形状?
-
Different forms, or allotropes, of carbon are diamond, graphite, and fullerenes.