1.1原子
章节大纲
-
Lesson Objectives
::经验教训目标- Describe atoms and how they are related to elements.
::描述原子及其与元素的关系。
- Identify the three main subatomic particles that make up atoms.
::识别组成原子的三大亚原子粒子。
Lesson Vocabulary
::词汇表课程- Atom
::原子原子
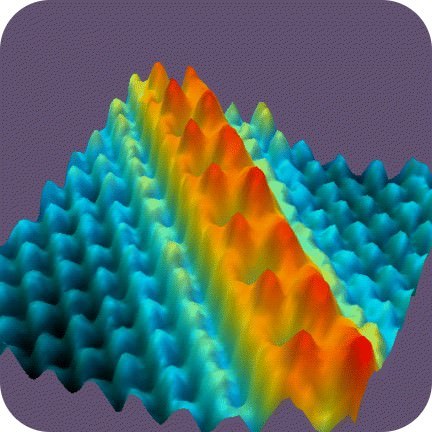
What could this hilly surface be? Do you have any idea? The answer is row upon row of atoms. To be specific, they are nickel atoms. The picture was created using a scanning tunneling microscope. No other microscope can make images of things as small as atoms. How small are atoms?
::山坡表面会是什么? 您知道吗? 答案是一排原子。 具体地说, 它们是镍原子。 图片是用扫描隧道显微镜制作的。 没有其他显微镜可以像原子那样小。 原子有多大?What Are Atoms?
::什么是原子? 原子吗? What are Atoms?Atoms are the building blocks of matter. Unlike blocks that we know, these building blocks are incredibly small. In fact, t hey are the smallest particles of an element. Atoms still have the same properties as the elements they make up. Elements are also pure substances. This means they are not mixed with anything else. Pure substances such as nickel, hydrogen, and helium make up all kinds of matter. All the atoms of a given element are identical. Atoms of different elements are not physically the same.
::原子是物质的构件。 与我们所知道的构件不同, 这些构件是极小的。 事实上, 它们是一个元素的最小粒子。 原子仍然具有与组成元素相同的特性。 元素也是纯物质。 这意味着它们没有与其他物质混合。 纯物质, 如镍、 氢和氦, 由各种物质组成。 某个元素的所有原子都是相同的。 不同元素的原子在物理上并不相同 。Think of something you might have made from LEGOs. You built some shape using the many different sized and shaped blocks. This is much like how atoms combine to become everything we know. If we took only one size and shape of block and put them together, we would make a pure substance. It would be an element. If you take apart anything that you have built, those individual parts are like the atoms. With those small parts, you build bigger things. Sometimes they are all the same type of block. Other times, they may be different kinds of blocks. We use these combinations of different blocks to make more complicated things.
::想想你可能从LEGOs中制造的东西。 你用许多不同大小和形状的区块构建了某种形状。 这就像原子如何结合成为我们所知道的一切。 如果我们只使用一个大小和形状的区块,然后把它们组合在一起, 我们就会形成一个纯物质。 它会是一个元素。 如果你拆分你所建造的东西, 这些个别部分就像原子。 这些小部分, 你就会建造更大的部分。 有时它们都是相同的区块类型。 有时, 它们可能是不同的区块。 我们用这些不同的区块组合来制造更复杂的东西 。Size of Atoms
::原子的大小Unlike LEGO bricks, atoms are extremely small. The radius of an atom is well under 1 nanometer. That's one-billionth of a meter. Such a number is hard to imagine. Consider this: trillions of atoms would fit inside the period at the end of this sentence. In other words, atoms are way too small to be seen with the naked eye.
::与LEGO砖块不同,原子是极小的。原子的半径远低于1纳米。 这是一米的10亿倍。 这个数字很难想象。 想想看: 数万亿原子将适合本句结尾的时段。 换句话说, 原子太小, 无法用肉眼看到 。Subatomic Particles
::亚原子粒子Although atoms are very tiny, they consist of even smaller particles. Atoms are made of protons, neutrons, and electrons:
::原子由质子、中子和电子组成:- Protons have a positive charge.
::质子的电量是正数
- Electrons have a negative charge.
::电子有负电荷
- Neutrons are neutral in charge.
::中中性中性中性负责。
Parts of the Atom
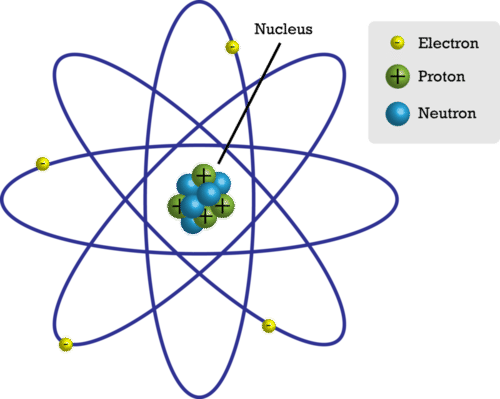
::原子部分Figure represents a simple model of an atom. Models help scientists make sense of things. Perhaps they are either too big or too small. Maybe they are just too complicated to make sense of. This simple model helps scientists think about the atom. Is this how the atom really looks? Not exactly! Remember, a model helps us make sense of things. They may not be an exact copy of the object. You will learn about more complex models of atoms in the coming years, but this model is a good place to start.
::图示是一个原子的简单模型。 模型帮助科学家理解事物。 也许它们太大或者太小。 也许它们太复杂了, 可能它们太复杂了。 这个简单模型帮助科学家思考原子。 这个简单模型是否帮助科学家思考原子的真面目? 不确切地说! 记住, 一个模型帮助我们理解事物。 它们可能不是物体的准确复制件。 你会在今后几年里了解更复杂的原子模型, 但是这个模型是一个良好的起始点。This simple atomic model shows the particles inside the atom.
::这个简单的原子模型显示原子内的粒子。The Nucleus
::核心At the center of an atom is the nucleus (plural, nuclei). The nucleus contains most of the atom’s mass. However, in size, it’s just a tiny part of the atom. The model in Figure is not to scale. If an atom were the size of a football stadium, the nucleus would be only about the size of a pea.
::原子的中心是核心(多元,核心 ) 。 核心包含大部分原子质量。 但是,从体积来看,它只是原子的一小部分。 图中的模型不是缩放。 如果原子是足球场的大小,核心将只有豆子的大小。The nucleus, in turn, consists of two types of particles, called protons and neutrons. These particles are tightly packed inside the nucleus. Constantly moving about the nucleus are other particles called electrons.
::核核反过来由两种粒子组成,称为质子和中子。这些粒子被紧紧地包在核中。在核中不断移动的其他粒子被称为电子。Protons
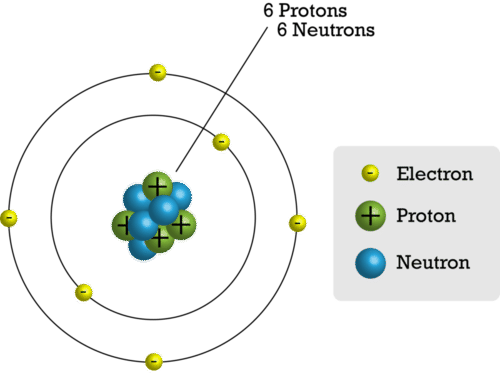
::质子A proton is a particle inside the nucleus of an atom. It has a positive electric charge. All protons are identical. It is all about the number of protons in the atoms. The number of protons is what gives the atoms of different elements their unique properties. Atoms of each type of element have a characteristic number of protons. For example, each atom of carbon has six protons (see Figure ). No two elements have atoms with the same number of protons.
::质子是原子核中的粒子。 它有正电荷。 所有质子都是相同的。 它都是关于原子中的质子数量。 质子的数量是赋予不同元素原子独特特性的质子数量。 每种元素的原子都有质子的特性数量。 例如, 每个原子的碳有6个质子(见图 )。 没有两个元素的原子与质子数量相同。Neutrons
::秒数A neutron is a particle inside the nucleus of an atom. It has no electric charge. Atoms of an element often have the same number of neutrons as protons. For example, most carbon atoms have six neutrons as well as six protons. This is also shown in Figure .
::中子是原子核中的粒子,没有电荷。元素原子的中子数量往往与质子相同。例如,大多数碳原子有6个中子和6个质子。图中也显示了这一点。This model shows the particles that make up a carbon atom.
::该模型显示组成碳原子的粒子。Electrons
::电An electron is a particle outside the nucleus of an atom. It has a negative electric charge. The charge of an electron is opposite but equal to the charge of a proton. Atoms have the same number of electrons as protons. As a result, the negative and positive charges "cancel out." This makes atoms electrically neutral. For example, a carbon atom has six electrons that "cancel out" its six protons.
::电子是原子核外的粒子。 它有负电荷。 电子的电荷与质子的电荷相反, 但与质子的电荷相等。 原子的电荷与质子的电荷相同。 结果, 负电荷和正电荷“ 取消 ” 。 这使得原子的电能保持中和。 例如, 碳原子有六种“ 解除” 质子的电能。Lesson Summary
::经验教训摘要- Atoms are the building blocks of matter. They are the smallest particles of an element. They still have the element's properties.
::原子是物质的构件。 它们是一个元素中最小的粒子。 它们仍然具有元素的属性 。
- All atoms are very small. Atoms of different elements vary in size.
::所有原子都很小,不同元素的原子大小不同。
- Three main types of particles that make up all atoms are protons, neutrons, and electrons.
::构成所有原子的三种主要颗粒类型是质子、中子和电子。
Lesson Review Questions
::经验回顾问题- What is an atom?
::什么是原子? 原子吗?
- Which of the following statements is true about the atoms of any element:
- They have the same number of protons as the atoms of all other elements.
::它们的质子数量与所有其他元素的原子数量相同。
- They have protons that are identical to the protons of all other elements.
::它们拥有与所有其他元素的质子相同的质子。
- They have the same size as the atoms of all other elements.
::它们与所有其他元素的原子大小相同。
- They have the same number of protons as neutrons.
::它们的质子数量与中子相同
::对于任何元素的原子,以下哪些语句是真实的:它们与所有其他元素的原子具有相同数量的质子。它们拥有与所有其他元素的质子相同的质子。它们拥有与所有其他元素的质子相同的质子。它们拥有与所有其他元素的原子相同的质子。它们拥有与中子相同的质子。 - They have the same number of protons as the atoms of all other elements.
- Describe atoms and how they are related to elements.