5.5 原子排放Spetra
Section outline
-
How much energy does it take to shoot an arrow?
::射箭需要多少能量?Archery as a sport or a means of defense has existed for centuries. At rest, there is no tension on the bowstring and no force on the arrow. When the string and arrow are pulled back, we now have a situation where (pulling of the string) has been converted to potential energy (the tension on the string). The archer releases the arrow, and the potential energy is translated into kinetic energy as the arrow moves. It turns out that electrons behave the same way when energy is put into the system or released from the system.
::箭术作为一种运动或防御手段已经存在了几个世纪。 在休息时,弓弦上没有紧张,箭头上也没有力量。当弦和箭头被拉回来时,我们现在有一种情况(弦的拉动)被转换为潜在的能量(弦的拉动 ) 。 弓箭手释放箭头,而潜在的能量随着箭头的移动而转化为动能。 事实证明,当能量被放入系统或从系统释放出来时,电子的行为也是一样的。Atomic Emission Spectra
::原子排放SpetraThe electrons in an tend to be arranged in such a way that the energy of the atom is as low as possible. The ground state of an atom is the lowest energy state of the atom. When those atoms are given energy, the electrons absorb the energy and move to a higher . These energy levels of the electrons in atoms are quantized, meaning again that the must move from one energy level to another in discrete steps rather than continuously. An excited state of an atom is a state where its potential energy is higher than the ground state. An atom in the excited state is not stable. When it returns back to the ground state, it releases the energy that it had previously gained in the form of electromagnetic radiation .
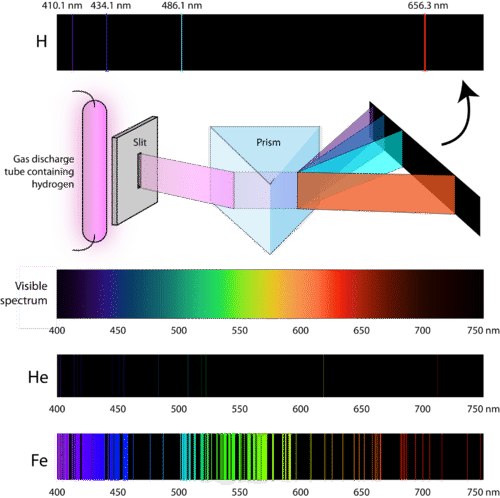
::电子的排列方式往往使原子的能量尽可能低。原子的地面状态是原子的最低能量状态。当这些原子得到能量时,电子吸收能量,并移动到更高的水平。原子中电子的能量水平被量化,这意味着原子的能量水平必须从一个能源水平转移到另一个能源水平,而不是不断地移动。原子的兴奋状态是其潜在能量高于地面状态的状态。振动状态中的原子不稳定。当它返回到地面状态时,它释放出它以前以电磁辐射的形式获得的能量。So how do atoms gain energy in the first place? One way is to pass an electric current through an enclosed sample of a at low pressure . Since the electron energy levels are unique for each , every gas discharge tube will glow with a distinctive color depending on the identity of the gas (see Figure ).
::原子最初是如何获得能量的? 一种方法是通过一个封闭的低压电流样本传递电流。 由于电子能量水平对每种气体来说都是独特的,每个气体排放管都会根据气体的特性以不同的颜色发光(见图 ) 。Gas discharge tubes are enclosed glass tubes filled with a gas at low pressure through which an electric current is passed. Electrons in the gaseous atoms first become excited, and then fall back to lower energy levels, emitting light of a distinctive color in the process. Shown are gas discharge tubes of helium, neon, argon, krypton, and xenon. “Neon” signs are familiar examples of gas discharge tubes. However, only signs that glow with the red-orange color seen in the figure are actually filled with neon. Signs of other colors contain different gases or mixtures of gases.
::“Neon”标志是气体排放管的常见例子,但是,只有用图中红色颜色发光的标志才实际填满了虹膜,其他颜色的标志含有不同的气体或气体混合物。Scientists studied the distinctive pink color of the gas discharge created by hydrogen gas. When a narrow beam of this light was viewed through a prism, the light was separated into four lines of very specific wavelengths (and frequencies since and are inversely related). An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The Figure shows the atomic emission spectrum of hydrogen.
::科学家们研究了氢气产生的气体排放的独特粉色。当通过棱镜观察这一光线的狭窄光束时,光线被分为四条非常具体的波长线(以及自和以来的频率反向相关 ) 。 原子排放频谱是光穿过棱镜将其分离成其所含不同光频率时形成的线条模式。图显示了氢原子排放频谱。When light from a hydrogen gas discharge tube is passed through a prism, the light is split into four visible lines. Each of these spectral lines corresponds to a different electron transition from a higher energy state to a lower energy state. Every element has a unique atomic emission spectrum, as shown by the examples of helium (He) and iron (Fe). Classical theory was unable to explain the existence of atomic emission spectra, also known as line-emission spectra. According to classical physics, a ground state atom would be able to absorb any amount of energy rather than only discrete amounts. Likewise, when the atoms relaxed back to a lower energy state, any amount of energy could be released. This would result in what is known as a continuous spectrum , where all wavelengths and frequencies are represented. White light viewed through a prism and a rainbow are examples of continuous spectra. Atomic emission spectra were more proof of the quantized nature of light and led to a new model of the atom based on quantum theory.
::古典理论无法解释原子排放光谱的存在,又称线性排放光谱。根据古典物理学,地面状态原子能够吸收任何能量,而不仅仅是离散能量。同样,当原子向低能量状态回落时,任何能量都可以释放出来。这将导致所谓的连续频谱,其中代表所有波长和频率。通过棱镜和彩虹观察的白光是连续光谱的例子。原子排放光谱更能证明光的量化性质,并导致基于量子理论的原子新模型。Look up at the sky at night and you'll see stars . How do we know what they are made of? Check out this simulation to explore atomic colors and the ingredients that make up our universe:
::晚上仰望天空,你会看到星星。我们怎么知道它们是由什么组成的?看看这个模拟,探索原子颜色和构成宇宙的元素:Summary
::摘要-
Atomic emission spectra are produced when excited electrons return to their ground state.
::当兴奋电子返回地面状态时,就会产生原子排放光谱。 -
The emitted light corresponds to energies of the specific electrons.
::发射的光与特定电子的能量相对应。
Review
::回顾-
What is the ground state of an atom?
::原子的地面状态如何? -
What is an excited state?
::什么是兴奋状态? -
Why do we see emission lines when electrons return to the ground state?
::当电子返回地面状态时,我们为什么看到排放线?
-
Atomic emission spectra are produced when excited electrons return to their ground state.