5.13 轨道轨道
Section outline
-
How is it that so many planes are able to fly without running into each other?
::为何这么多的飞机 能在不相撞的情况下飞行?The flight path of a commercial airliner is carefully regulated by the Federal Aviation Administration. Each airplane must maintain a distance of five miles from another plane flying at the same altitude and 2,000 feet above and below another aircraft (1,000 feet if the altitude is less than 29,000 feet). So, each aircraft only has certain positions it is allowed to maintain while it flies. As we explore , we see that electrons have similar restrictions on their locations.
::商业客机的飞行路线由联邦航空管理局仔细管理。每架飞机必须保持与另一架飞机5英里的距离,以同一高度飞行,在另一架飞机上方和下方2 000英尺(如果高度低于29 000英尺,则为1 000英尺)。因此,每架飞机只有某些位置,在飞行时可以维持。我们看到电子在其位置上也有类似的限制。Orbitals
::轨道轨道We can apply our knowledge of to describe the arrangement of electrons for a given . We do this with something called configurations . They are effectively a map of the electrons for a given atom. We look at the four quantum numbers for a given electron and then assign that electron to a specific orbital.
::我们可以运用我们的知识来描述给定对象的电子安排 。 我们用所谓的配置来做。 它们实际上是给定原子电子的地图 。 我们查看给定电子的四量数数字, 然后将电子分配到特定的轨道 。s Orbitals
::s 轨道轨道For any value of , a value of places that electron in an s orbital . This orbital is spherical in shape:
::对于 n 的任何值, 值为 l=0 的值将电子置于轨道中。 此轨道的形状是球形 :p Orbitals
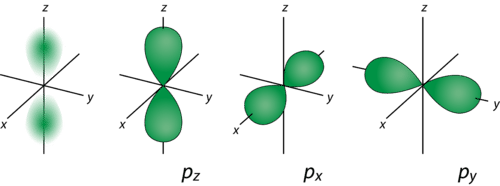
::p 轨道轨道From Table we see that we can have three possible orbitals when . These are designated as p orbitals and have dumbbell shapes. Each of the p orbitals has a different orientation in three-dimensional space.
::从表中可以看出,当l=1时,我们可以有三种可能的轨道。这些轨道被指定为轨道,有哑铃形状。每个轨道在三维空间都有不同的方向。d Orbitals
::d轨道轨道When , values can be -2, -1, 0, +1, +2 for a total of five d orbitals . Note that all five of the orbitals have specific three-dimensional orientations.
::当 l= 2, ml 值可以是 - 2, - 1, 0, +1, +2, 共为 5 d 轨道。 请注意, 所有 5 个轨道都有特定的三维方向 。f Orbitals
::轨道轨道The most complex set of orbitals are the f orbitals . When , values can be -3, -2, -1, 0, +1, +2, +3 for a total of seven different orbital shapes. Again, note the specific orientations of the different f orbitals.
::最复杂的轨道是轨道轨道。当 l=3, ml 值可以是 -3, -2, -1, 0, +1, +2, +2, +3, 共七个不同的轨道形状。 请注意不同轨道轨道的具体方向 。Electron Arrangement Within Energy Levels Principal Quantum Number "> Allowable Sublevels Number of Orbitals per Sublevel Number of Orbitals per Principal Number of Electrons per Sublevel Number of Electrons per Principal Energy Level 1 s 1 1 2 2 2 s
::s , s , s , s , s , s , s , s , sp
::p p p ) pp1
3
4 2
6
8 3 s
::s , s , s , s , s , s , s , s , sp
::p p p ) ppd
::d , d , d , d , d , d , d , d , d , d , d1
3
5
9
2
6
10
18
4 s
::s , s , s , s , s , s , s , s , sp
::p p p ) ppd
::d , d , d , d , d , d , d , d , d , d , df
::f , f , f , f1
3
5
7
16
2
6
10
14
32
Summary
::摘要-
There are four different classes of electron orbitals.
::有四类不同的电子轨道。 -
These orbitals are determined by the value of angular momentum quantum number
.
::这些轨道由角动量量量数 1 的价值决定。
Review
::回顾-
What is an electron configuration?
::什么是电子配置? -
How many electrons are in the
orbital?
::n=1轨道上有多少电子? -
What is the total number of electrons in a p orbital?
::轨道上的电子总数是多少? -
How many electrons does it take to completely fill a d orbital?
::完全填满轨道需要多少个电子?
-
There are four different classes of electron orbitals.