6.9 氢和阿尔卡利金属
Section outline
-
Can you guess what kind of reaction is taking place in this picture?
::你能猜到这张照片里有什么反应吗?Some chemistry students just enjoy learning about the science, while others are intrigued by the violent reactions that sometimes can occur. Many chemistry classes have been enlivened by the demonstration of how reactive sodium is with water. In some instances, the demonstration has gone off safely. Unfortunately, in other situations students and instructors have incurred serious injury due to their failure to observe proper safety precautions.
::有些化学系学生只是喜欢学习科学,而另一些学生则对有时可能发生的暴力反应感到好奇。 许多化学系班都因为水中的钠反应力的示范而生动。 在有些情况下,示威活动安全地结束了。 不幸的是,在另一些情况下,学生和教官由于未能遵守适当的安全防范措施而受到严重伤害。One value of the periodic table is the ability to make predictions about the behavior of individual . By knowing which group an element is in, we can determine the number of reactive electrons and say something about how that element will behave.
::周期表的一个值是能够对个人的行为做出预测。通过知道元素在哪个组中,我们可以确定反应电子的数量,并对该元素的行为方式发表一些看法。Hydrogen and Alkali Metals
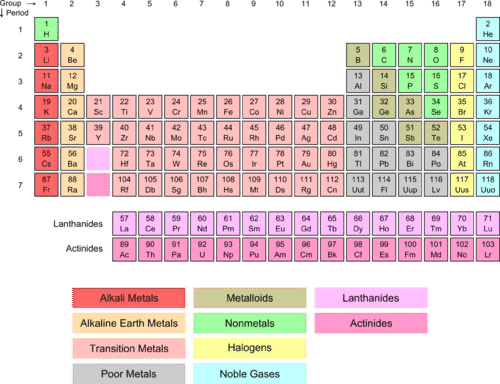
::氢和阿尔卡利金属The periodic table is arranged on the basis of (number of in the nucleus). One of the valuable consequences of this arrangement is that we can learn a lot about the distribution in these atoms. The colors in the table below indicate the different groupings of atoms based on the location and number of electrons in the .
::周期表是根据(核心数)排列的。这一安排的一个宝贵后果是我们可以了解这些原子的分布。下表的颜色显示根据原子的位置和电子数量的不同原子组别。If we look at Group I (red column), we see that it is labeled alkali metals . Also note the green H above the alkali metals. All of these elements have a similar configuration of outer-shell electrons (see Table ). In each case, there is one electron in the outer and that is an s -orbital electron. Hydrogen is not an alkali metal itself, but has some similar properties due to its simple one proton ( located in the nucleus), one electron arrangement. The lone electron exists in a s -orbital around the nucleus . For lithium, there are two 1 s electrons in an inner orbit and one 2 s electron in the outer orbit. The same pattern holds for sodium and potassium.
::如果我们看一组(红柱),我们可以看到它是有标签的碱金属。请注意碱金属上方的绿色H,所有这些元素的外壳电子结构相似(见表 )。在每种情况下,外壳中都有一个电子,即S-轨道电子。氢本身不是碱金属,但由于其简单的一个质子(位于核内),一个电子安排,具有一些相似的特性。单电子存在于核心周围的轨道。对于锂而言,内轨道有两枚电子,外轨道有两枚电子。钠和钾的情况也是如此。Element
::要素要素Symbol
::符号符号符号Electron Configuration
::电子配置hydrogen
::氢氢H
::赫 时1 s 1
::1 秒1lithium
::锂Li
::李李[He]2 s 1
::[他]2s1sodium
::钠Na
::纳纳[Ne]3 s 1
::[新]3s1potassium
::钾K
::K K 级[Ar]4 s 1
::[阿 4s1rubidium
::Rb
::卢比[Kr]5 s 1
::[Kr]5s1cesium
::Cs
::C 数[Xe]6 s 1
::[Xe]6s1francium
::Fr
::中 调[Rn]7 s 1
::[Rn]7s1Even an atom with a very complex electron composition such as cesium still has the single s electron in its outer orbital (see Figure ).
::即使一个原子具有非常复杂的电子成分,例如,其外轨道上仍有单一电子(见图 )。Cesium Orbitals. This one electron is very easily removed during . The group I elements react rapidly with oxygen to produce metal oxides. They are very soft metals, which become just above room temperature .
::一种电子在 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 第一 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 一 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Li reacts with water to produce hydrogen . Sodium also reacts the same way, just more rapidly. Potassium reacts rapidly with water producing hydrogen gas and which ignites the hydrogen gas. Rubidium and cesium react yet more vigorously and explode on contact with water.
::硅与水发生反应产生氢。钠也发生相同反应,反应速度更快。钾与产生氢气的水反应迅速,并点燃氢气。和反应更加激烈,与水接触时爆炸。Summary
::摘要-
Group I (alkali metals and hydrogen) elements all have one electron in their outer shell. This electron is in a s orbital.
::第一类(碱金属和氢)元素的外壳都有一个电子,这种电子在轨道上。 -
The Group I metals are all very reactive with water.
::第一组金属都对水非常有反应。
Review
::回顾-
What group are the alkali metals and hydrogen in?
::碱金属和氢是哪些组的? -
What is the outer shell electron configuration in this group?
::这个组的外壳电子配置是什么? -
How reactive are the alkali metals with oxygen?
::碱金属的氧气反应如何? -
How reactive are these metals with water?
::这些金属对水的反应如何?
-
Group I (alkali metals and hydrogen) elements all have one electron in their outer shell. This electron is in a s orbital.