7.1 分子公式
章节大纲
-
Why are music notes a unique "language"?
::为什么音乐是独一无二的"语言"?There are many “universal languages” in the world. Musicians of every culture recognize the music embodied in a series of notes on a staff.
::世界上有许多“通用语言 ” , 每一种文化的音乐家都承认在工作人员的一系列笔记中体现的音乐。This passage from a Bach cello suite could be played by any trained musician from any country, because there is agreement as to what the symbols on the page mean. In the same way, molecules are represented using symbols that all chemists agree upon.
::巴赫大提琴套房的这一通道可由来自任何国家的受过训练的音乐家演奏,因为对页面上的符号的含义有一致意见。 同样,分子使用所有化学家都同意的符号来表示。Molecular Formula
::分子公式A molecule is two or more atoms that have been chemically combined. A molecular formula is a of a molecular that shows the kinds and numbers of atoms present in a molecule of the compound. Ammonia is a compound of nitrogen and hydrogen as shown below:
::分子是化学上结合的两种或两种以上的原子。分子式是一种分子,显示化合物分子中存在的原子的种类和数量。氨是氮和氢的化合物,如下文所示:Note from the example that there are some standard rules to follow in writing molecular formulas. The arrangements of the depend on the particular structure, so we will not concern ourselves with that point right now. The number of atoms of each kind is indicated by a subscript following the . If there is only one atom, no number is written. If there is more than one atom of a specific kind, the number is written as a subscript following the atom. We would not write N 3 H for ammonia, because that would mean that there are three nitrogen atoms and one hydrogen atom in the molecule, which is incorrect.
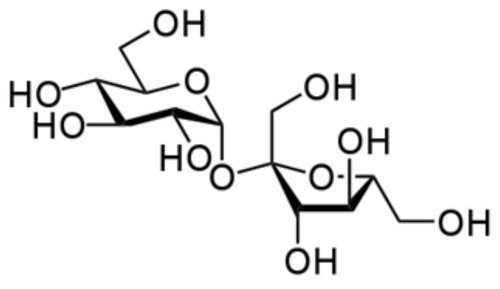
::请注意,在笔记分子公式方面有一些标准规则。 取决于特定结构的安排, 因此我们现在不会关注这一点。 每种原子的数量由下面的下标标表示。 如果只有一个原子, 则不写数字。 如果有一个以上特定类型的原子, 数字则在原子之后写成下标。 我们不会为氨写N3H, 因为这意味着分子中有三个氮原子和一个氢原子, 这是错误的 。The molecular formula does not tell us anything about the shape of the molecule or where the different atoms are. The molecular formula for sucrose (table sugar) is C 12 H 22 O 11 . This simply tells us the number of carbon, hydrogen, and oxygen atoms in the molecule. There is nothing said about where the individual atoms are located. We need a much more complicated formula (shown below) to communicate that information.
::分子公式没有告诉我们分子的形状或不同原子的位置。 sucrosse (可溶糖) 的分子公式是 C12H22O11。 这只告诉我们分子中的碳、 氢和氧原子的数量。 没有说明单个原子的位置。 我们需要更复杂的公式( 如下表) 来传递这些信息 。Summary
::摘要-
A molecular formula tells us what atoms and how many of each type of atom are present in a molecule.
::分子公式告诉我们分子中存在哪些原子和多少种原子。 -
If only one atom of a specific type is present, no subscript is used.
::如果只有一个特定类型的原子存在,则不使用下标。 -
For atoms that have two or more present, a subscript is written after the symbol for that atom.
::对于存在两个或两个以上的原子,在原子符号之后写上下标。 -
Molecular formulas do not indicate how the atoms are arranged in the molecule.
::分子式没有表明原子是如何在分子中排列的。
Review
::回顾-
What does a molecular formula tell us?
::分子公式告诉我们什么? -
What does a molecular formula not tell us?
::分子公式没有告诉我们什么? -
What do the subscripts mean in a molecular formula?
::在分子公式中,下标意味着什么? -
If you wrote C
6
H
11
O
5
C
6
H
11
O
6
as the molecular formula for sucrose, would that be correct? Explain your answer.
::如果你把C6H11O5C6H11O6作为苏格罗斯的分子公式写成 C6H11O5C6H11O6,这是否正确? -
Sometimes the formula for acetic acid is written CH
3
COOH. Is this a true molecular formula?
::有时乙酸的配方是CH3COOH的写法。这是真的分子配方吗?
-
A molecular formula tells us what atoms and how many of each type of atom are present in a molecule.