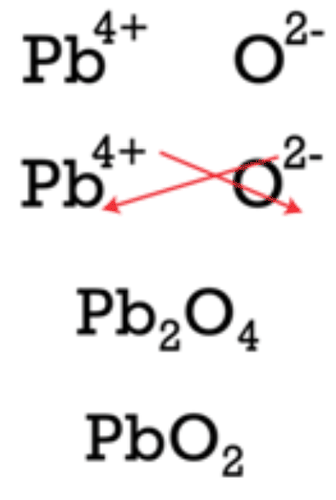7.8 二元离子化合物公式
章节大纲
-
How does shorthand work?
::速记如何工作?Shorthand was a very popular way of recording speech, especially in dictating letters and in court testimony. Instead of trying to write out all the words, the person taking the dictation would use a set of symbols that represented syllables or words. The pages above show a shorthand version of “A Christmas Carol” written by Charles Dickens. Unless you know shorthand, the passage is meaningless. But knowing shorthand allows you to read this classic story.
::短手是一种非常流行的录音演讲方式,特别是在写字和法庭证词时。 听字的人不但没有试图写出所有词,反而会使用代表音节或文字的一套符号。 上面的页面展示了查尔斯·狄更斯写的“圣诞卡罗尔”的短手版本。 除非您知道短手,否则这一段是毫无意义的。 但是,知道短手可以让你读到这个经典故事。Different professions also use a type of shorthand in communication to save time. Chemists use chemical symbols in combination to indicate specific compounds. There are two advantages to this approach:
::化学家们使用化学符号组合来表示具体的化合物。-
The
under discussion is clearly described so there can be no confusion about its identity.
::正在讨论的内容已清楚说明,因此不能混淆其特性。 -
Chemical symbols represent a universal language that all chemists can understand, no matter what their native language is.
::化学符号代表着一种所有化学家都能理解的普遍语言,
Writing Formulas for Binary Ionic Compounds
::二进音离音化合物的写法公式If you know the name of a binary ionic compound , you can write its chemical formula . Start by writing the metal with its charge, followed by the ion with its charge. Because the overall compound must be electrically neutral, decide how many of each ion is needed in order for the positive and negative charge to cancel each other out. Consider the compound aluminum nitride. The ions are:
::如果您知道二进制离子化合物的名称, 您可以写入其化学配方 。 从刻上金属充电开始, 之后是离子充电。 由于整个化合物必须是电中性, 请决定每离子需要多少个, 才能取消正负电荷。 考虑化合物铝硝化物。 离子是:
::Al3+N3-Since the ions have charges that are equal in magnitude, one of each will be the lowest ratio of ions in the formula. The formula of aluminum nitride is AlN.
::由于电离子的电荷在数量上相等,其中之一将是公式中离子比率最低的。铝硝酸盐的公式是AlN。The ions for the compound lithium oxide are:
::化合物锂氧化物的离子是:
::里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 - 里加O2 -In this case, two lithium ions are required to balance out the charge of one oxide ion. The formula of lithium oxide is Li 2 O.
::在这种情况下,需要两个锂离子来平衡一个氧化离子的电荷,氧化锂的公式是Li2O。An alternative way to writing a correct formula for an is to use the crisscross method. In this method, the numerical value of each of the ion charges is crossed over to become the subscript of the other ion. Signs of the charges are dropped. Shown below is the crisscross method for aluminum oxide.
::写一个正确公式的替代办法是使用晶体十字路口法。在这个方法中,每种离子电荷的数值被跨过,成为另一个离子的下标。降低电荷的符号。下面显示的是氧化铝的晶体十字路口法。The red arrows indicate that the 3 from the 3+ charge will cross over to become the subscript of the O. The 2 from the 2− charge will cross over to become the subscript of the Al. The formula for aluminum oxide is Al 2 O 3 .
::红色箭头显示,3+填充的3将交叉成为O的下标。 2-填充的2将交叉成为Al的下标。氧化铝的公式是 Al2O3。Be aware that ionic compounds are empirical formulas and so must be written as the lowest ratio of the ions. In the case of aluminum nitride, the crisscross method would yield a formula of Al 3 N 3 , which is not correct. It must be reduced to AlN. Following the crisscross method to write the formula for lead(IV) oxide would involve the following steps:
::请注意,离子化合物是实验性公式,因此必须写成离子的最低比率。在铝硝酸盐的情况下,晶体交错法产生Al3N3的公式,该公式不正确。该公式必须降为AlN。The crisscross first yields Pb 2 O 4 for the formula, but that must be reduced to the lower ratio and PbO 2 is the correct formula.
::克里斯克首先得出公式的Pb2O4,但必须降低到较低的比率,而PbO2是正确的公式。Summary
::摘要-
Formulas for binary compounds begin with the metal followed by the non-metal.
::二元化合物的公式从金属开始,然后是非金属。 -
Positive and negative charges must cancel each other out.
::正面和负面收费必须相互取消。 -
Ionic compound formulas are written using the lowest ratio of ions.
::电离离子复合公式采用离子比例最低的方法写成。
Review
::回顾-
Write formulas for the binary ionic compounds formed between the following pairs of elements:
-
cesium and fluorine
::和氟 -
calcium and sulfur
::钙和硫磺 -
aluminum and chlorine
::铝和氯 -
zinc and nitrogen
::锌和氮
::在以下几对元素之间形成的二氧化离子化合物的写公式: 和氟钙、硫铝、氯锌和氮。 -
cesium and fluorine
-
Write the formula and give the name for the compound formed by the following ions:
-
Fe
3+
and O
2-
::FE3+和O2和O2 -
Ni
2+
and S
2-
::Ni2+和S2和S2 -
Au
+
and Cl
-
::Au+和Cl - -
Sn
4+
and I
-
::Sn4+和I -
::写入公式并给出由以下离子组成的化合物的名称:Fe3+和O2-Ni2+和S2-Au+和Cl-Sn4+和I- -
Fe
3+
and O
2-
-
Give names for the following compounds:
-
Ag
2
S
::AG2S( 阿格2S) -
PdO
::PdO PdO -
PtCl
4
::PtCl4 点Cl4 -
V
2
O
5
::V2O5
::给出下列化合物的名称: AG2S PdO PtCl4 V2O5 -
Ag
2
S
-
The
under discussion is clearly described so there can be no confusion about its identity.