9.23 混合轨道轨道 -- -- 螺和螺和螺和螺
章节大纲
-
How do you open the closed circle?
::你如何打开封闭的圆圈?Romeo and Juliet were two of the great lovers of all time. Their embrace allowed no other person to be a part of it – they only wanted to be with each other. It took outside intervention (parents are like that!) to get them away from one another. Paired electrons are similar to the lovers. They do not bond covalently until they are unpaired. Then they can become a part of a larger chemical structure.
::罗密欧和朱丽叶是历史上两个伟大的情人。他们的拥抱不允许其他人参与其中,他们只想彼此相伴。他们需要外界的干预(父母就是这样 ) , 才能让他们彼此分离。 美人电子和情人相似。 在他们被遗忘之前,他们没有共同的纽带。 然后,他们就可以成为更大的化学结构的一部分。Hybrid Orbitals – sp and sp 2
::混合轨道轨道-sp和sp2sp Hybridization
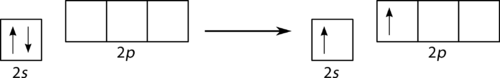
::双平衡化A beryllium hydride (BeH 2 ) molecule is predicted to be linear by VSEPR. The beryllium contains all paired electrons and so must also undergo hybridization . One of the 2s electrons is first promoted to the empty 2p x (see Figure ).
::VSEPR预测氢化(BeH2)分子为线性。包含所有配对电子,因此也必须进行混合。其中之一首先被推广到空的2px(见图 )。Promotion of Be 2s electron.
::推广二等电子。Now the hybridization takes place only with the occupied orbitals and the result is a pair of sp hybrid orbitals . The two remaining p orbitals ( p y and p z ) do not hybridize and remain unoccupied (see Figure ).
::现在,混合化只与所占领的轨道发生,结果产生一对螺旋混合轨道。 剩下的两颗轨道轨道(py和pz)不进行混合并仍然无人使用(见图 )。Be hybrid orbitals.
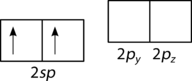
::成为混合轨道。The geometry of the sp hybrid orbitals is linear, with the lobes of the orbitals pointing in opposite directions along one axis, arbitrarily defined as the x-axis (see Figure ). Each can bond with a 1s orbital from a hydrogen atom to form the linear BeH 2 molecule.
::螺旋混合轨道的几何是线性的,轨道的叶子朝相反方向沿着一个轴,任意定义为x轴(见图 ) 。每个轴与氢原子的1秒轨道相连接,形成线性BEH2分子。The process of sp hybridization is the mixing of an s orbital with a single p orbital (the pxorbital by convention), to form a set of two sp hybrids. The two lobes of the sp hybrids point opposite one another to produce a linear molecule.
::螺旋混合的过程是将一个轨道与一个单轨道(按公约的毛轨道)混合起来,形成一组由两个样形混合体组成的一组。Other molecules whose electron domain geometry is linear and for whom hybridization is necessary also form sp hybrid orbitals. Examples include CO 2 and C 2 H 2 , which will be discussed in further detail later.
::其他分子的电子域几何为线性,必须进行混合处理,这些分子也构成螺旋混合轨道,例如CO2和C2H2,将在以后进一步详细讨论。sp 2 Hybridization
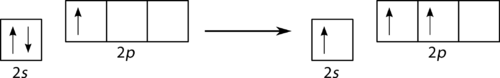
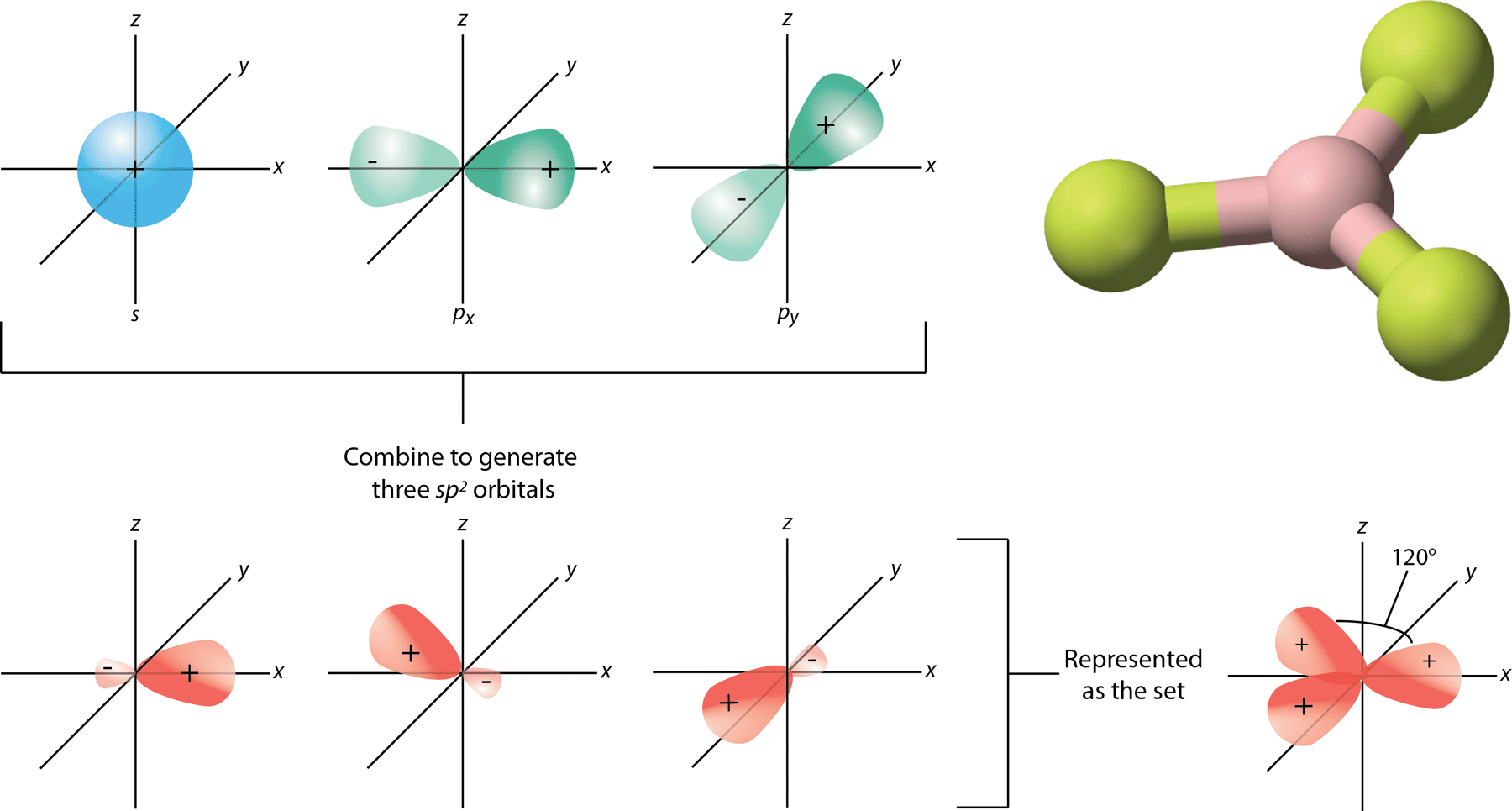
::spsp2 混合化Boron trifluoride (BF 3 ) is predicted to have a trigonal planar geometry by VSEPR. First a paired 2s is promoted to the empty 2p y orbital (see Figure ).
::三氟化(BF3)预计将有VSEPR的三角平面几何测量法。 首先,将对对2推进到空的2平轨道(见图 )。Promotion of 2s electron.
::推广2s电子。This is followed by hybridization of the three occupied orbitals to form a set of three sp 2 hybrids, leaving the 2p z orbital unhybridized (see Figure ).
::随后是三个已占据轨道的混合化,形成一套由三个SB2混合体组成的组合体,使2pz轨道的轨道未加环绕(见图 )。Formation of sp 2 orbital.
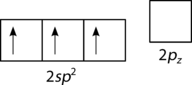
::形成Sp2轨道轨道。The geometry of the sp 2 hybrid orbitals is trigonal planar, with the lobes of the orbitals pointing towards the corners of a triangle (see Figure ). The angle between any two of the hybrid orbital lobes is 120°. Each can bond with a 2 p orbital from a fluorine atom to form the trigonal planar BF 3 molecule.
::螺旋2混合轨道的几何是三角平面,轨道的叶子指向三角形的角(见图 ) 。 任何两个混合轨道叶之间的角为 120 °。 每种轨道都可以与氟原子的2p轨道相连接,形成三角平面 BF3 分子。The process of sp 2 hybridization is the mixing of an s orbital with a set of two p orbitals (p x and p y ) to form a set of three sp 2 hybrid orbitals. Each large lobe of the hybrid orbitals points to one corner of a planar triangle.
::螺旋2混合化的过程是将一个轨道与一套两颗轨道(px和py)混合,形成一套由三颗螺旋2混合轨道组成的系统,混合轨道的每个大叶子都指向一个平面三角形的一个角。Other molecules with a trigonal planar electron domain geometry form sp 2 hybrid orbitals. Ozone (O 3 ) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen makes the molecular geometry bent. The hybridization of the central O atom of ozone is sp 2 .
::臭氧(O3)是一个分子的例子,其电子域几何为三角平面平面平面平面平面平面平面平面平面,尽管中央氧中存在单对,分子几何轨道倾斜。Summary
::摘要-
Paired electrons can be hybridized and then participate in covalent bonding.
::等离子电子可以混合,然后参与共价结合。
Review
::回顾-
Does the ground state beryllium atom contain any unpaired electrons?
::地表是否含有任何未受污染的电子? -
Why does one
2s
electron in Be get promoted to a
2p
orbital?
::为什么Be的一二电子 会被提升到2P轨道? -
What is the geometry of the two sp orbitals?
::两个螺旋轨道的几何形状是什么?
-
Paired electrons can be hybridized and then participate in covalent bonding.