13.18 供暖和冷却曲线
章节大纲
-
How is it that steamboats contain so much power?
::蒸汽船怎么会有这么大的动力?During the time of Mark Twain (real name Samuel Langhorne Clemens, 1835-1910), the steamboat was a major means of transportation on the rivers and lakes of the United States. Twain himself was a steamboat pilot on the Mississippi River for a period of time and took his pen name from the measurement of water depth (twelve feet, which was a safe depth for the boats). The boats got their power from steam – water converted to a at high temperatures. The steam would push the pistons of the engine, causing the paddle wheels to turn and propel the boat.
::在Mark Twain(1835-1910年,原名Samuel Langhorne Clemens , 1835-1910年)期间,蒸汽船是美国河流和湖泊的主要运输工具。 Twain本人曾是密西西西比河的汽艇飞行员,在一段时间内从水深测量中取用他的笔名(12英尺,对船只来说是安全的深水 ) 。 船舶的动力来自蒸汽 — — 水在高温下转换为高温。 蒸汽会推动发动机的支架,导致轮轮转向和推进船。Heating Curves
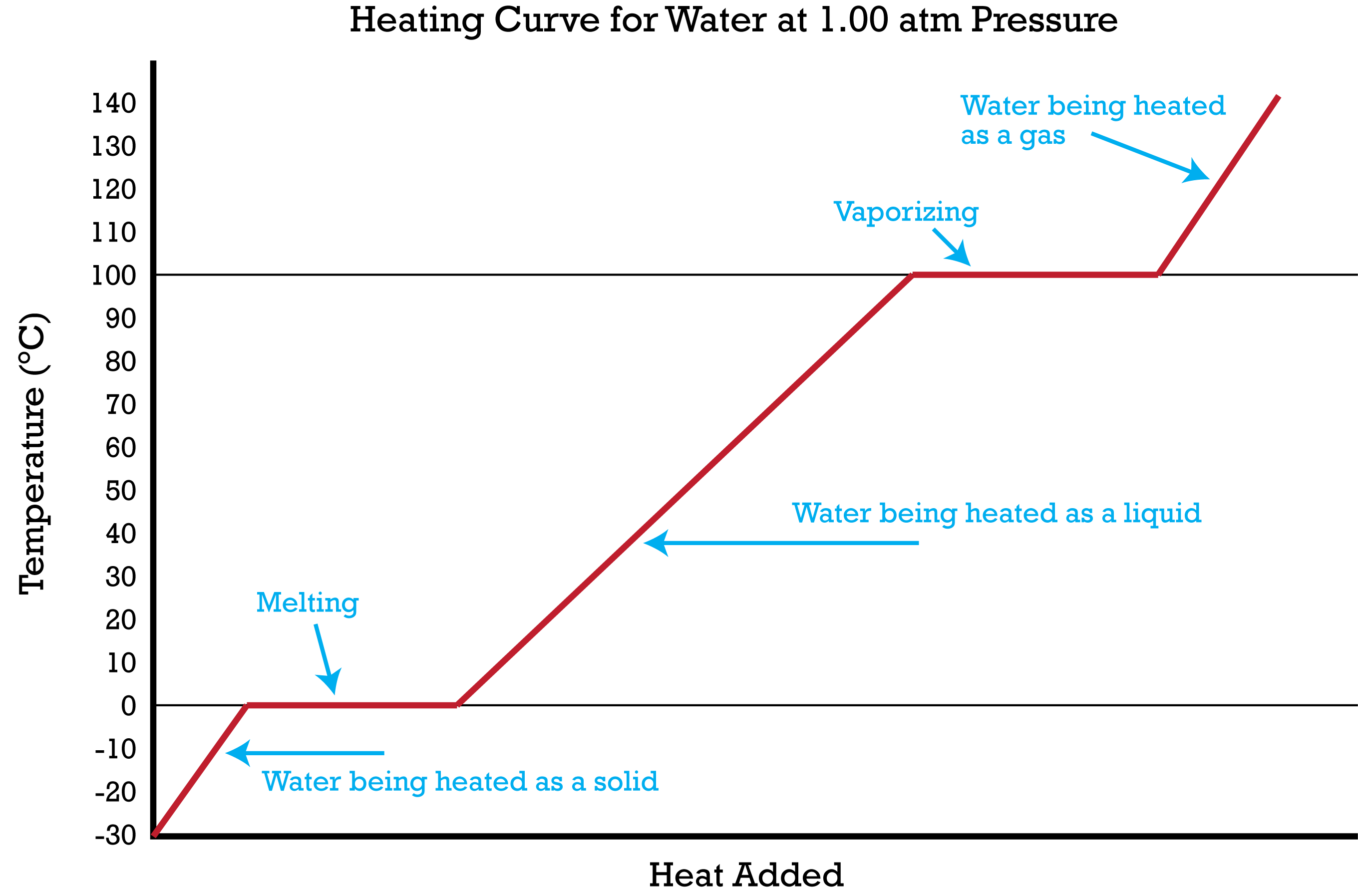
::供暖曲线Imagine that you have a block of ice that is at a temperature of -30°C, well below its point. The ice is in a closed container. As is steadily added to the ice block, the water molecules will begin to vibrate faster and faster as they absorb . Eventually, when the ice has warmed to 0°C, the added energy will start to break apart the that keeps the water molecules in place when it is in the solid form. As the ice melts, its temperature does not rise. All of the energy that is being put into the ice goes into the melting process and not into any increase in temperature. During the melting process, the two states – solid and liquid – are in equilibrium with one another. If the system was isolated at that point and no energy was allowed to enter or leave, the ice-water at 0°C would remain. Temperature is always constant during a .
::想象一下,你有一块冰块,温度在 - 30°C, 远低于其点。 冰在封闭的容器中。 与冰块中稳步添加的一样,水分子将随着吸收而开始加速振动。 最终, 当冰温升至零°C时, 增加的能量将开始分裂保持水分子的状态, 当它以坚固的形式存在时。 随着冰融化, 它的温度不会上升。 所有注入冰中的能量都会进入融化过程, 而不是任何温度的上升。 在融化过程中, 两个州 — — 固体和液体 — — 都处于平衡之中。 如果当时的系统被隔开,没有能量被允许进入或离开, 零°C的冰水将保持不变。 在一个冰融化过程中,温度总是保持不变。Continued heating of the water after the ice has completely melted will now increase the kinetic energy of the liquid molecules and the temperature will rise. Assuming that the is standard, the temperature will rise steadily until it reaches 100°C. At this point, the added energy from the heat will cause the liquid to begin to vaporize. As with the previous state change, the temperature will remain at 100°C while the water molecules are going from the liquid to the gas or vapor state. Once all the liquid has completely boiled away, continued heating of the steam (remember the container is closed) will increase its temperature above 100°C.
::冰完全融化后,水的继续加热将增加液体分子的动能,温度将上升。假设是标准温度,温度将稳步上升,直到达到100°C。此时,热增加的能量将使液体开始蒸发。与先前的状态变化一样,温度将保持在100°C,而水分子则从液体到气体或蒸气状态。一旦所有液体完全沸腾,蒸汽的继续加热(记得容器关闭后)将把温度提高到100°C以上。The described above can be summarized in a graph called a heating curve ( Figure ):
::上述情况可以用一个称为加热曲线(图)的图表加以概括:In the heating curve of water, the temperature is shown as heat is continually added. Changes of state occur during plateaus because the temperature is constant.
::在水的加热曲线中,温度显示为连续加热,高原期间状态变化是因为温度不变。The change of state behavior of all substances can be represented with a heating curve of this type. The melting and boiling points of the substance can be determined by the horizontal lines or plateaus on the curve. Other substances would of course have melting and boiling points that are different from those of water. One exception to this exact form for a heating would be for a substance such as carbon dioxide which sublimes rather than melts at standard pressure . The heating curve for carbon dioxide would have only one plateau, at the temperature of CO 2 .
::所有物质的状态行为变化可以用这种类型的加热曲线来表示。物质的熔点和沸点可以由曲线上的水平线或高原来决定。其他物质当然会有不同于水的熔点和沸点。这种加热形式的一个例外是二氧化碳等物质在标准压力下沉降而不是融化。二氧化碳的加热曲线在二氧化碳温度下只有一个高点。The entire experiment could be run in reverse. Steam above 100°C could be steadily cooled down to 100°C, at which point it would condense to liquid water. The water could then be cooled to 0°C, at which point continued cooling would freeze the water to ice. The ice could then be cooled to some point below 0°C. This could be diagrammed in a cooling curve that would be the reverse of the heating curve.
::整个实验可以倒流进行。 超过100°C的蒸气可以稳定地冷却到100°C, 当它凝结到液态水时, 水可以冷却到0°C, 继续冷却会将水冷却到冰层。 然后冰可以冷却到零°C以下的某个点。 这可以用一个冷却曲线来图解, 冷却曲线将是加热曲线的反方向 。Summary of State Changes
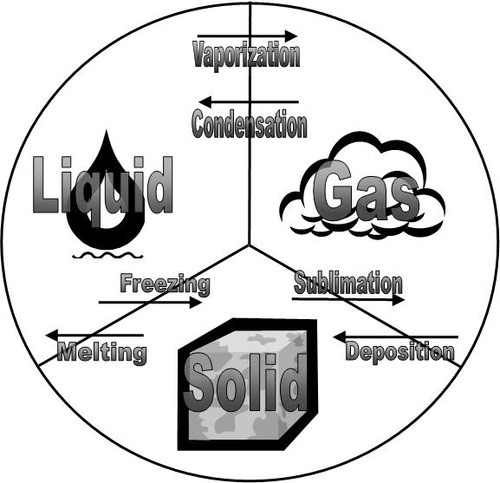
::国家变化摘要All of the that occur between solid, liquid and gas are summarized in the diagram in the figure below. is the opposite of melting and both represent the equilibrium between the solid and liquid states. Vaporization occurs when a liquid turns to a gas. is the opposite of vaporization and both represent the equilibrium between the liquid and gas states. is the opposite of sublimation and both represent the equilibrium between the solid and gas states.
::固体、液体和气体之间发生的一切情况在下图的图表中加以归纳。两者均代表固体和液体状态之间的平衡。当液体转向气体时发生蒸发作用。两者均代表液态和气体状态之间的平衡。两者均代表升降的相反情况,两者均代表固体和气体状态之间的平衡。Solid, liquid, and gas states with the terms for each change of state that occurs between them.
::固态、液态和气体状态,以及它们之间每次发生状态变化的条件。Summary
::摘要-
A change of state can be brought about by putting heat into a system or removing it from the system.
::向一个系统加热或从系统中去除热能,可以带来国家的变化。 -
The temperature of a system will not change as long as the substance is undergoing a change from solid to liquid or liquid to gas, as well as the reverse.
::只要物质正在从固体变成液体或液体变成气体,以及反向变化,一个系统的温度就不会改变。
Review
::回顾-
What happens when ice reaches 0°C?
::当冰到达0°C时会怎样? -
What is sublimation?
::什么是升华? -
What happens to steam if it is cooled to 100°C?
::如果冷却到100°C 蒸汽会怎么样?
-
A change of state can be brought about by putting heat into a system or removing it from the system.