16.8 解决百分率
章节大纲
-
How many is too much?
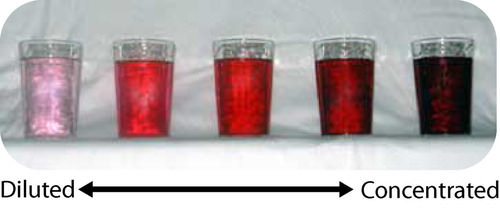
::有多少太多了?There are cultures that have no numbers above three. Anything greater than that is simply referred to as “much” or “many”. We recognize how limited this form of calculation is, but we do some of the same thing. There are several ways to express the amount of solute in a solution in a quantitative manner. The of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution is one that has a relatively small amount of dissolved solute. However, those terms are vague and we need to be able to express concentration with numbers.
::有的文化数量不超过三个以上。任何比这更简单称为“大”或“许多”的文化。我们认识到这种计算方式有多么有限,但我们做了一些同样的事情。有几种方法可以用数量表示溶液的溶液数量。一种解决办法是测量溶液数量,溶解溶解的溶液数量。一种集中的解决办法是溶解溶液数量相对较多的溶解溶液。一种稀释的解决方法是溶解溶液数量相对较少的溶解溶液。然而,这些术语含糊不清,我们需要能够用数量表示集中。Percent Solutions
::百分率解决方案One way to describe the concentration of a solution is by the percent of a solute in the solvent. The percent can further be determined in one of two ways: (1) the ratio of the mass of the solute divided by the mass of the solution or (2) the ratio of the volume of the solute divided by the volume of the solution.
::一种描述溶液集中程度的方法是溶液溶液溶液中溶液的百分率。 可以用两种方式之一进一步确定该百分率1)溶液质量除以溶液质量的比例,或者(2)溶液体积除以溶液体积的比例。
Mass Percent
::质量百分比When the solute in a solution is a solid, a convenient way to express the concentration is a mass percent ( mass mass ) , which is the grams of solute per 100 g of solution.
::当溶液溶液中溶液是固体时,表示浓度的方便方式是质量百分比( massmass),即每100克溶液溶液溶液溶液溶液的克。Percent by mass = mass of solute mass of solution × 100 %
::按质量=溶液溶液溶液的溶液质量百分比x100%Suppose that a solution was prepared by dissolving 25.0 g of sugar into 100 g of water. The percent by mass would be calculated by:
::假设将25.0克糖溶解到100克水中,就可以形成一种解决办法。Percent by mass = 25 g sugar 125 g solution × 100 % = 20 % sugar
::按质量=25克糖125克溶液x10020%糖计算的百分比Sometimes you may want to make up a particular mass of solution of a given percent by mass and need to calculate what mass of the solvent to use. For example, you need to make 3000 g of a 5% solution of sodium chloride. You can rearrange and solve for the mass of solute.
::有时,您可能想要用质量来计算给定百分比的某一质量的溶液, 并且需要计算溶剂的重量。 例如, 您需要将氯化钠的溶液量定在 5% 的 3000 克。 您可以重新排列和解析溶液的重量 。mass of solute = percent by mass 100 % × mass of solution = 5 % 100 % × 3000 g = 150 g NaCl
::溶液质量100兆克=5% 1003000克=150克NaClYou would need to weigh out 150 g of NaCl and add it to 2850 g of water. Notice that it was necessary to subtract the mass of the NaCl (150 g) from the mass of solution (3000 g) to calculate the mass of the water that would need to be added.
::您需要测量150克NaCl, 并将其加到2850克的水中。 请注意, 需要从溶液质量( 3000克)中减去纳Cl质量( 150克) , 以计算需要添加的水的质量 。Volume Percent
::音量百分比The percentage of solute in a solution can more easily be determined by volume when the are both . The volume of the solute divided by the volume of solution expressed as a percent yields the percent by volume ( volume volume ) of the solution. If a solution is made by adding 40 mL of ethanol to 200 mL of water, the percent by volume is:
::溶液溶液在溶液中的百分比,如果两者都是以体积来决定,则更容易根据体积来确定。溶液的体积除以以百分比表示的溶液量来按体积(体积)来得出溶液的百分率。如果将乙醇的40毫升加到200毫升的水量来解决这个问题,则按体积计算的百分比如下:Percent by volume = volume of solute volume of solution × 100 % = 40 mL ethanol 240 mL solution × 100 % = 16.7 % ethanol
::按体积分列的百分比=溶液溶液溶液溶液的溶液容量x10040毫升乙醇240毫升溶液x10016.7%乙醇Frequently, ingredient labels on food products and medicines have amounts listed as percentages (see Figure ).
::食品和药品的成分标签经常以百分比列出(见图)。Hydrogen peroxide is commonly sold as a 3% by volume solution for use as a disinfectant.
::过氧化氢通常按体积溶液销售3%,用作消毒剂。Summary
::摘要-
Techniques for calculation percent mass and percent volume solution concentrations are described.
::介绍了计算质量百分比和体积溶液浓度百分比的技术。
Review
::回顾-
Why are the terms “concentrated” and “dilute” not very helpful?
::为什么“集中”和“细化”这两个术语没有多大帮助? -
When would you use volume percent calculations?
::你什么时候会用量的百分率计算? -
Why do you subtract the mass of the solute from the total mass desired when making mass percent calculations?
::在计算质量百分比时,为什么从总质量中减去溶液的质量?
-
Techniques for calculation percent mass and percent volume solution concentrations are described.