17.14 国家变动的多步问题
Section outline
-
Which takes more heat – melting or boiling?
::这需要更多热量 — — 熔化还是沸腾?You have a cube of ice. Which process will take more energy – the of that ice cube or the conversion of the water to steam? The short answer is that more energy is needed to convert the water to steam. The long answer is really a question: how do you get from one point to the other? What is the temperature of the ice? What is the mass of that ice cube? A lot goes into taking the material from the starting point to the end-point.
::你有一块冰块。哪个过程需要更多的能量 — — 冰块的能量或者将水转换为蒸汽?简短的答案是需要更多的能量来将水转换为蒸汽。 长期的答案其实是一个问题:如何从一个点到另一个点?冰块的温度是多少?冰块的重量是多少?从开始点到终点的原料的含量是多少?Multi-Step Problems with Changes of State
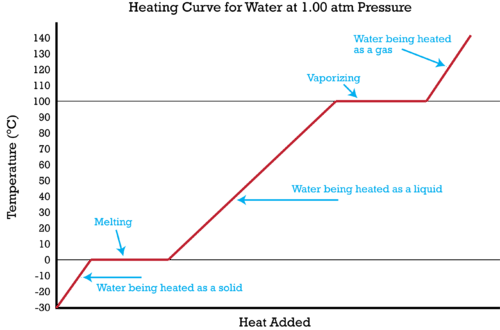
::国家变动的多步问题Heating curves show the phase changes that a substance undergoes as is continuously absorbed.
::供暖曲线显示物质随着不断吸收而经历的阶段变化。Heating curve of water.
::水的加热曲线The specific heat of a substance allows us to calculate the heat absorbed or released as the temperature of the substance changes. It is possible to combine that type of problem with a to solve a problem involving multiple steps. Figure shows ice at -30°C being converted in a five-step process to gaseous water (steam) at 140°C. It is now possible to calculate the heat absorbed during that entire process. The process and the required calculation is summarized below.
::物质的具体热量使我们能够随着物质温度的变化而计算吸收或释放的热量,可以将这种类型的问题与解决涉及多个步骤的问题结合起来。图显示的是-30°C的冰正在以五步过程转换成140°C的气体水(气流)。现在可以计算整个过程吸收的热量。下面将概述这一过程和所要求的计算结果。-
Ice is heated from -30°C to 0°C. The heat absorbed is calculated by using the specific heat of ice and the equation
.
::吸收的热量通过使用特定冰的热量和QH=cp×mT等式计算。 -
Ice is melted at 0°C. The heat absorbed is calculated by multiplying the
moles
of ice by the
molar heat of fusion
.
::冰在0°C时熔化。吸收的热量通过将冰的摩尔数乘以聚变的摩尔热来计算。 -
Water at 0°C is heated to 100°C. The heat absorbed is calculated by using the specific heat of water and the equation
.
::0°C的水被加热到100°C。吸收的热量通过使用特定的热水和等式QH=cp×mT计算。 -
Water is vaporized to steam at 100°C. The heat absorbed is calculated by multiplying the moles of water by the
molar heat of vaporization
.
::吸收热量的计算方法是,将水的摩尔数乘以蒸发的摩尔热。 -
Steam is heated from 100°C to 140°C. The heat absorbed is calculated by using the specific heat of steam and the equation
.
::蒸气从100°C加热到140°C。 吸收的热量通过使用特定的蒸汽热和QH=cp×mT等式计算。
Sample Problem: Multi-Step Problems using a Heating Curve
::样本问题:使用加热曲线的多步问题Calculate the total amount of heat absorbed (in kJ) when 2.00 mol of ice at -30.0°C is converted to steam at 140.0°C. The required specific heats can be found in the table in " Heat Capacity and Specific Heat".
::计算热吸收总量(以千焦耳计),当 -3.0.0°C为 -3.0.0°C的2.00 毫升冰转化为140.0°C的蒸汽时。 所需的特定热量可在“热能和特定热量”的表格中找到。Step 1: List the known quantities and plan the problem .
::第1步:列出已知数量并规划问题。Known
::已知已知-
2.00 mol ice = 36.04 g ice
::2.00毫升冰 = 36.04克冰 -
::cp(冰)=2.06 J/gC -
:水)=4.18 J/gC
-
::cp(蒸汽)=1.87 J/gC -
::*Hfus=6.01 kJ/mol -
::@Hvap=40.7 kJ/mol
Unknown
::未知-
::总和=?
Follow the steps previously described. Note that the mass of the water is needed for the calculations that involve the specific heat, while the moles of water is needed for the calculations that involve . All heat quantities must be in kilojoules so that they can be added together to get a total for the five-step process.
::遵循前述步骤。 请注意, 涉及特定热量的计算需要水的质量, 而涉及特定热量的计算则需要水的摩尔。 所有热量都必须在千焦耳中, 才能将它们加在一起, 才能得出五步过程的总和 。Step 2: Solve .
::步骤2:解决。-
::*H1=2.06 J/gCx36.04 gx30*Cx1 kJ1000 J=2.23 kJ -
::*H2=2.00 molx6.01 kJ1 mol=12.0 kJ -
::H3=4.18 J/gC×36.04 gx100C×1 kJ1000 J=15.1 kJ -
::*H4=2.00 molx40.7 kJ1 mol=81.4 kJ -
::*H5=1.87 J/gCx36.04 gx40*Cx1 kJ1000 J=2.70 kJ
::H1H2H3H4H5=113.4千焦耳Step 3: Think about your result .
::步骤3:想想你的结果。The total heat absorbed as the ice at -30°C is heated to steam at 140°C is 113.4 kJ. The largest absorption of heat comes during the vaporization of the water.
::30°C的冰层在140°C时加热蒸汽时吸收的总热量为113.4千焦耳。 最大吸收热量是在水蒸发期间吸收的。Summary
::摘要-
Multi-step calculations for changes of state are described.
::说明了国家变化的多步计算。
Review
::回顾-
Why are two different sets of units used?
::为什么使用两套不同的单元? -
What other units problem do you need to be aware of?
::你还需要了解其他单位的问题吗? -
What would you need to know to do calculations like this for acetone?
::你要知道什么才能计算丙酮?
-
Ice is heated from -30°C to 0°C. The heat absorbed is calculated by using the specific heat of ice and the equation
.