18.4 潜在能源图
章节大纲
-
What was Sisyphus’s punishment?
::西西弗斯的惩罚是什么?Sisyphus was a mythological being who had been a very evil king. As a punishment, he was supposed to roll a large stone up to the top of a long hill. A spell had been placed on the stone so that it would roll back down before reaching the top, so the task could never be completed . Sisyphus was condemned to an eternity of trying to get to the top of the hill, but never succeeding.
::西西弗斯是一个神话中的神话人物,他曾经是一个非常邪恶的国王。作为一种惩罚,他应该把一块大石头卷到一个长山顶上。 石块被施了咒语,这样它就会在爬上山顶之前倒下来,这样任务就永远无法完成。 西西弗斯注定永远要试图爬上山顶,但永远不能成功。Potential Energy Diagrams
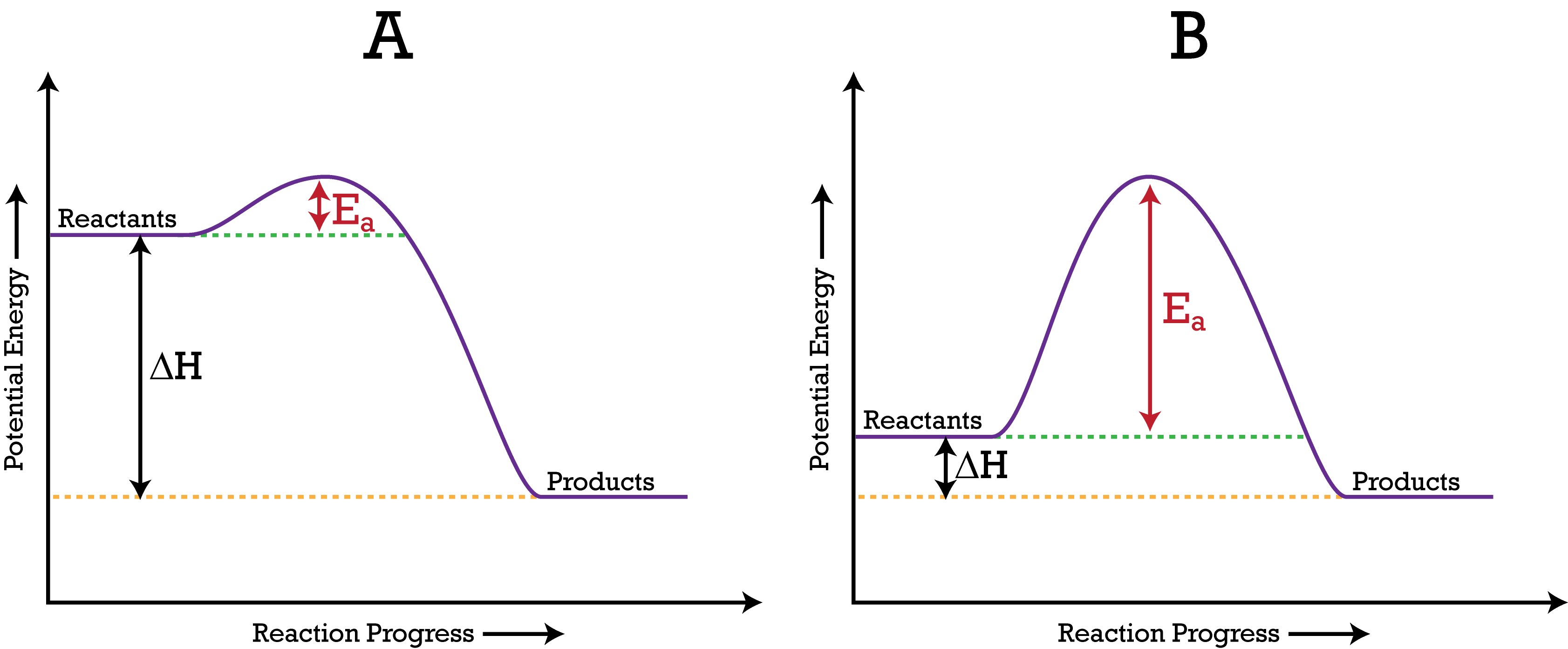
::潜在能源图The energy changes that occur during a can be shown in a diagram called a potential energy diagram , or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Figure shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the change is positive for an and negative for an . This can be seen in the potential energy diagrams. The total potential energy of the system increases for the endothermic reaction as the system absorbs energy from the surroundings . The total potential energy of the system decreases for the exothermic reaction as the system releases energy to the surroundings.
::在一种称为潜在能源图的图表中,或有时称为反应进度曲线的图表中,可以显示在一种情况下发生的能量变化。潜在的能源图显示当反应剂转换成产品时,一个系统的潜在能量变化。图显示的是终端热反应(A)和异温反应(B)的基本潜在能量图表。回顾这一变化(H)对一种能量是正的,对一种能量是负的。这可以从潜在的能源图中看出。随着系统吸收周围的能量,这个系统的总潜在能量会增加其末温反应。当系统向周围释放能量时,这个系统的总潜在能量会减少对异温反应的能量。A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ΔH is negative.
:A) 在局部热反应中,产品的能量大于反应者的能量,H是正数。 (B) 在异热反应中,产品的能量低于反应者的能量,H是负数。
The for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products. For this reason, the activation energy of a reaction is sometimes referred to as the activation energy barrier. Reacting particles must have enough energy so that when they collide they can overcome that barrier (see Figure ).
::反应所需的能量在潜在能量图中以反应物和产品之间的山峰高度来说明。 因此,反应的活能有时被称为激活能量屏障。 反应粒子必须有足够的能量,以便它们相撞时能够克服这个屏障(见图 )。The activation energy (E a ) of a reaction is the barrier that must be overcome for the reactants to be able to become products. (A) The activation energy is low, meaning that the reaction is likely to be fast. (B) The activation energy is high, meaning that the reaction is likely to be slow.
::反应的活化能量(Ea)是反应者成为产品必须克服的障碍。 (A) 激活能量低,这意味着反应可能很快。 (B) 激活能量高,意味着反应可能缓慢。Summary
::摘要-
Potential energy diagrams for endothermic and exothermic reactions are described.
::介绍了内温和异温反应的潜在能量图。 -
Diagrams of activation energy and reaction progress are given.
::提供了激活能量和反应进展的图表。
Review
::回顾-
In an endothermic reaction, is the potential energy of the products higher or lower than the potential energy of the reactants?
::在局部热反应中,这些产品的潜在能量是否高于或低于反应机的潜在能量? -
In an exothermic reaction, is the potential energy of the products higher or lower than the potential energy of the reactants?
::在异热反应中,这些产品的潜在能量是否高于或低于反应机的潜在能量? -
What does activation energy tell us?
::激活能量告诉我们什么?
-
Potential energy diagrams for endothermic and exothermic reactions are described.