18.9 反应令
Section outline
-
How harmful are forest fires?
::森林火灾有多有害?Forest fires cause extensive damage when they occur. Both plant and animal life are harmed during these events. The severity of a forest fire depends on how much plant life is available to burn – the more available dry plant material, the more serious the fire and the more rapidly it will spread.
::森林火灾一旦发生,就会造成巨大破坏。 在这些事件中,动植物生命都会受到伤害。 森林火灾的严重性取决于有多少植物生命可以燃烧 — — 有多少干燥植物材料,火灾越严重,其蔓延速度就越快。Order of Reaction
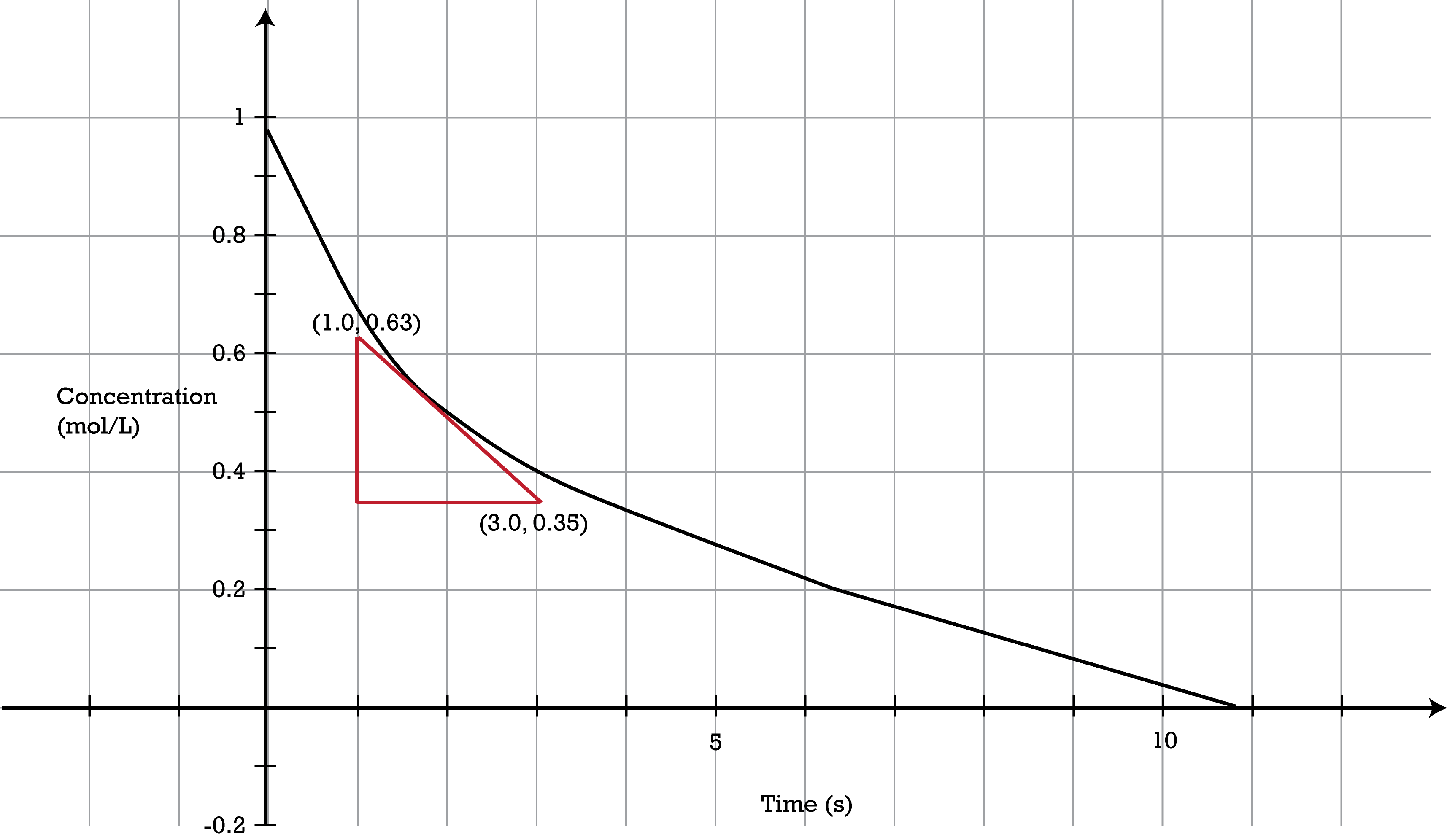
::反应顺序In the reaction , the rate of the reaction is directly proportional to the of raised to the first power. That is to say, . A first-order reaction is a reaction in which the rate is proportional to the concentration of only one reactant . As a first-order reaction proceeds, the rate of reaction decreases because the concentration of the reactant decreases (see Figure ). The graph of concentration versus time is curved. The can be determined graphically by the slope of a tangent to the curve at any point. The rate of the reaction at the time shown with the red triangle is given by:
::在AB反应中,反应速率与A升至第一级反应的速率直接成比例。也就是说,[A]=[A]1]。第一级反应是该速率与只有一个反应器的集中度成比例的反应。作为第一级反应的收益,反应速率由于反应器的集中性下降而下降(见图 )。集中度与时间的图是曲线的曲线。([A]}}}(t)可以通过相切值与曲线的任何点的斜坡以图形方式确定。显示红色三角形时的反应速率如下:
::==0.14 m/sThis graph shows how the concentration of a reactant changes as a reaction proceeds. The rate of the reaction is determined at any point by measuring the slope of a tangent to the curve. The rates of some reactions depend on the concentrations of more than one reactant. Consider a reaction in which a molecule of collides with a molecule of to form product .
::一些反应的速率取决于不止一个反应剂的浓度,考虑A分子与B分子相撞形成产品C的一种反应。
::A+B_C+B_C+B_C+B_C+B_C+B_C+B_C+B_C+C+B_C+B_C+C+B_C+B_C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C_C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+C+CDoubling the concentration of alone would double the reaction rate. Likewise, doubling the concentration of alone would also double the rate. The rate law must reflect the rate dependence on both reactants.
::将A的集中程度翻一番是反应率的两倍。 同样,将B的集中程度翻一番也将是B的两倍。 比率法必须反映对两个反应者的比率依赖性。
::速率=k[A][B]This reaction is said to be first order with respect to and first order with respect to . Overall, it is a second-order reaction. The rate law and the order of a reaction must be determined experimentally.
::据说这种反应对A是第一级,对B是第一级。 总的来说,这是第二级反应,比率法和反应顺序必须实验性地确定。Summary
::摘要-
A first-order reaction is described.
::描述的是第一级反应。
Review
::回顾-
What is a first-order reaction?
::什么是一阶反应? -
How is the instantaneous rate determined?
::瞬时费率是如何确定的? -
How do we determine the rate law and reaction order?
::我们如何确定比例法和反应秩序?
-
A first-order reaction is described.