2.8 水的生化特性 -- -- 先进
Section outline
-
What may be the most important molecule for life?
::生命中最重要的分子是什么?Some may argue . Some may argue certain . But many would argue . And what makes water so important? Its properties. The nature of the three atoms and how they interact with each other. This allows water to be a polar molecule, which allows it to interact with many other molecules necessary for life. Most of the substances in a are floating around in a water-based cytoplasmic environment.
::有些人可能会争论。 有些人可能会争论肯定。 但许多人会争论。 是什么让水如此重要? 水的特性。 三个原子的性质及其相互作用方式。 这让水成为极分子, 从而可以与生命所需的许多其他分子互动。 在一个物质中, 大部分物质都漂浮在以水为基础的细胞结构环境中。Chemical Structure and Properties of Water
::水的化学结构和特性You are probably already familiar with many of water’s properties. For example, you no doubt know that water is tasteless, odorless, and transparent. In small quantities, it is also colorless. However, when a large amount of water is observed, as in a lake or the ocean, it is actually light blue in color. The blue hue of water is an intrinsic property and is caused by selective absorption and scattering of white light. These and other properties of water depend on its chemical structure.
::您可能已经熟悉了水的许多特性。 比如,您无疑知道水是无品味、无味、透明的。 少量水也是无色的。 但是,当观测到大量水时,比如湖或海洋中,水的颜色实际上是浅蓝色的。 蓝色的水是内在的特性,是由白光选择性吸收和散射造成的。 水的这些和其他特性取决于水的化学结构。The transparency of water is important for organisms that live in water. Because water is transparent, sunlight can pass through it. Sunlight is needed by water plants and other water organisms for .
::水的透明度对于生活在水中的生物很重要,因为水是透明的,阳光可以穿透水面。 水厂和其他水生物需要阳光。Chemical Structure of Water
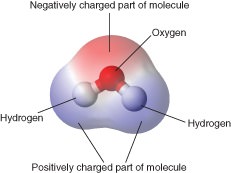
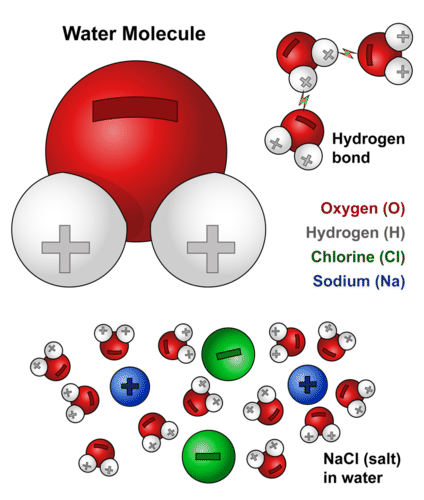
::水的化学结构Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical formula H 2 O. The arrangement of atoms in a water molecule, shown in the Figure , explains many of water’s chemical properties. In each water molecule, the of the oxygen atom (with 8 positively charged protons) attracts electrons much more strongly than do the hydrogen nuclei (with only one positively charged proton). This results in a negative electrical charge near the oxygen atom (due to the "pull" of the negatively charged electrons toward the oxygen nucleus) and a positive electrical charge near the hydrogen atoms. A difference in electrical charge between different parts of a molecule is called polarity . A polar molecule is a molecule in which part of the molecule is positively charged and part of the molecule is negatively charged.
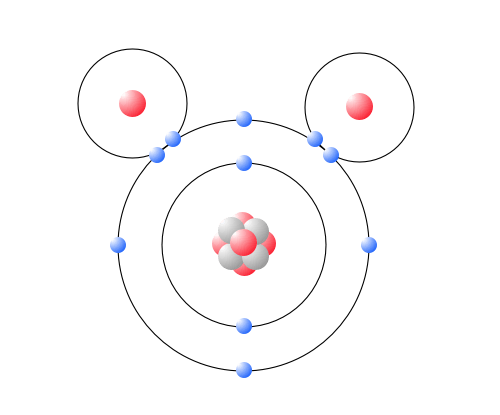
::每个水分子由一个氧原子和两个氢原子组成,因此它拥有化学公式H2O。图中显示的水分子中的原子安排解释了水的许多化学特性。在每个水分子中,氧原子(有8个正充电质子)比氢核(只有一个正充电质子)更能吸引电子。这导致氧原子附近的负电荷(由于对氧核负电荷的“拉动”)和氢原子附近的正电荷。一个分子的不同部分的电荷差异被称为极性。一个极分子是一种分子,其中分子的一部分是正充电,分子的一部分是负电。This model shows the arrangement of oxygen and hydrogen atoms in a water molecule. A water molecule has a bent or angular (non-linear) shape, with an angle of about 105°. The nucleus of the oxygen atom attracts electrons more strongly than do the hydrogen nuclei. As a result, the middle part of the molecule near oxygen has a negative charge, and the other parts of the molecule have a positive charge. In essence, the electrons are "pulled" toward the nucleus of the oxygen atom and away from the hydrogen atom nuclei. Water is a polar molecule, with an unequal distribution of charge throughout the molecule. This model is an atomic diagram of water, showing the two hydrogen atoms and an oxygen atom in the center. The protons and neutrons (red and grey) are in the center (nucleus) of each atom, and the electrons (blue) circle each nucleus. Not all of the nuclear particles found in oxygen are shown here. This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can interact with a water molecule. Hydrogen Bonding
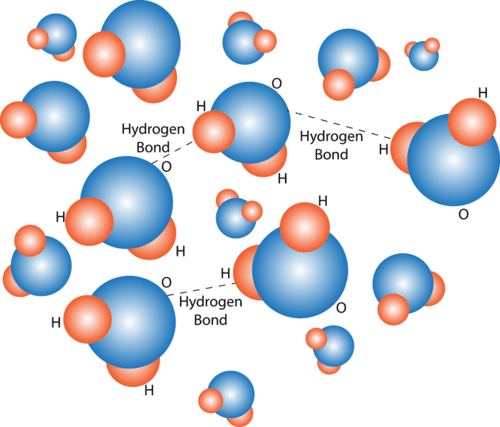
::氢联结Opposite electrical charges attract one another. Therefore, the positive part of one water molecule is attracted to the negative parts of other water molecules. Because of this attraction, bonds form between hydrogen and oxygen atoms of adjacent water molecules, as demonstrated in the Figure . This type of bond always involves a hydrogen atom, so it is called a hydrogen bond . Hydrogen bonds are bonds between molecules, and they are not as strong as bonds within molecules. Nonetheless, they help hold water molecules together.
::对面电荷相互吸引。 因此, 一种水分子的正分子被其他水分子的负分子吸引。 由于这种吸引力, 相邻水分子的氢原子和氧原子之间形成了联系, 如图所示。 这种联系总是涉及氢原子, 因而被称为氢联结。 氢联结是分子之间的联结, 它们没有分子内部的联结那么强大。 尽管如此, 它们有助于将水分子凝聚在一起。Hydrogen bonds form between positively and negatively charged parts of water molecules. The bonds hold the water molecules together. How do you think this might affect water’s properties? Hydrogen bonds can also form within a single large organic molecule . For example, hydrogen bonds that form between different parts of a protein molecule bend the molecule into a distinctive shape, which is important for the protein’s functions. Hydrogen bonds also hold together the two nucleotide chains of a DNA molecule.
::氢离子也可以形成于单一的大型有机分子中。 比如,在蛋白分子不同部分之间形成的氢离子会将分子变成一种独特的形状,这对于蛋白质的功能很重要。 氢离子也会将DNA分子的两个核酸链条连在一起。Sticky, Wet Water
::粘性,湿水Water has some unusual properties due to its hydrogen bonds. One property is cohesion , the tendency for water molecules to stick together. The cohesive forces between water molecules are responsible for the phenomenon known as surface tension . The molecules at the surface do not have other like molecules on all sides of them and consequently, they cohere more strongly with those directly associated with them on the surface. For example, if you drop a tiny amount of water onto a very smooth surface, the water molecules will stick together and form a droplet, rather than spread out over the surface. The same thing happens when water slowly drips from a leaky faucet. The water doesn't fall from the faucet as individual water molecules but as droplets of water. The tendency of water to stick together in droplets is also illustrated by the dew drops in the Figure .
::水的特性之一是凝固,水分子的倾向是凝固。水分子之间的凝固力是造成表面紧张现象的原因。地表上的分子没有其他像分子一样的分子,因此,它们与地面上直接相连的分子聚集得更加紧密。例如,如果将一小撮水滴到非常平滑的表面,水分子就会凝固在一起形成一个滴子,而不是在地表上扩散。同样的情况是,水从漏水的水流中缓慢滴水。水不会作为个别的水分子而作为水滴从水流中滴出。水流中粘结在一起的倾向也通过图中的水滴来说明。Droplets of dew cling to a spider web, demonstrating cohesion, the tendency of water molecules to stick together because of hydrogen bonds. Another important physical property of water is adhesion . In terms of water, adhesion is the bonding of a water molecule to another substance, such as the sides of a leaf's veins . This process happens because hydrogen bonds are special in that they break and reform with great frequency. This constant rearranging of hydrogen bonds allows a percentage of all the molecules in a given sample to bond to another substance. This grip-like characteristic that water molecules form causes capillary action , the ability of a liquid to flow against gravity in a narrow space. An example of capillary action is when you place a straw into a glass of water. The water seems to climb up the straw before you even place your mouth on the straw. The water has created hydrogen bonds with the surface of the straw, causing the water to adhere to the sides of the straw. As the hydrogen bonds keep interchanging with the straw's surface, the water molecules interchange positions and some begin to ascend the straw.
::水的另一个重要物理特性是粘合。在水方面,粘合是水分子与另一种物质(如叶叶的侧面)的结合。这一过程之所以发生,是因为氢联结是特别的,因为氢联结破裂和非常频繁地进行改造。氢联结的不断重新排列使得特定样本中所有分子的一定比例能够与另一种物质结合。水分子形成这种紧凑的特征会导致毛细作用,液体在狭小的空间中对重力流动的能力。一个毛细作用的例子就是把吸管放在水杯中。水似乎爬上稻草,甚至把嘴放在稻草上。水与稻草表面形成了氢联结,使水粘在稻草边上。随着氢联结与稻草表面的交织,水分子的交替位置和一些人开始升起稻草。Adhesion and capillary action are necessary to the survival of most organisms. It is the mechanism that is responsible for water transport in plants through and and in through small .
::多数生物的生存需要附着和毛细行动,这是负责通过小植物和通过小植物和小植物进行水运的机制。Hydrogen bonds also explain why water’s boiling point (100°C) is higher than the boiling points of similar substances without hydrogen bonds. Because of water’s relatively high boiling point, most water exists in a liquid state on Earth. Liquid water is needed by all living organisms. Therefore, the availability of liquid water enables life to survive over much of the planet.
::氢债券也解释了为什么水的沸点(100°C)高于没有氢键的类似物质的沸点。 由于水的沸点相对较高,大部分水存在于地球上的液体状态中。 所有活生物体都需要液态水。 因此,液态水的供给使地球上大部分地区的生命得以生存。Furthermore, water has a high specific heat because it takes a lot of energy to raise or lower the temperature of water. As a result, water plays a very important role in temperature regulation. Since cells are made up of water, this property helps to maintain .
::此外,水具有高特有热量,因为它需要大量能量才能提高或降低水的温度。 因此,水在温度调节中起着非常重要的作用。 由于细胞由水组成,这种特性有助于维持。Density of Ice and Water
::冰和水的密度The melting point of water is 0°C. Below this temperature, water is a solid (ice). Unlike most chemical substances, water in a solid state has a lower density than water in a liquid state. This is because water expands when it freezes. Again, hydrogen bonding is the reason. Hydrogen bonds cause water molecules to line up less efficiently in ice than in liquid water. As a result, water molecules are spaced farther apart in ice, giving ice a lower density than liquid water. A substance with lower density floats on a substance with higher density. This explains why ice floats on liquid water, whereas many other solids sink to the bottom of liquid water.
::水的熔点是 0 °C 。 在此温度下, 水是固体( 冰) 。 与大多数化学物质不同, 固态水的密度比液态水低。 这是因为水在冷冻时会膨胀。 同样, 氢联结也是原因。 氢联结导致水分子在冰中排成比液态水效率低。 因此, 水分子在冰中间隔更远, 冰的密度比液态水低。 密度较低的物质在密度较高的物质上漂浮。 这解释了为什么在液态水上会浮冰, 而其他许多固体会沉入液态水底。In a large body of water, such as a lake or the ocean, the water with the greatest density always sinks to the bottom. Water is most dense at about 4°C. As a result, the water at the bottom of a lake or the ocean usually has a temperature of about 4°C. In climates with cold winters, this layer of 4°C water insulates the bottom of a lake from freezing temperatures. Lake organisms such as can survive the winter by staying in this cold, but unfrozen, water at the bottom of the lake.
::在大体水中,如湖泊或海洋,密度最大的水总是沉入海底,水密度最高,大约为4°C。因此,湖底或海洋的水通常温度约为4°C。在冬季寒冷的气候下,4°C的这一层水使湖底免受冰冷的温度的侵扰。在寒冷、解冻的湖泊底部的水中,这种4°C的湖生物可以活过冬天。Summary
::摘要-
Water molecules are polar, so they form hydrogen bonds. This gives water unique properties, such as a relatively high boiling point, high specific heat, cohesion, adhesion and density.
::水分子是极极分子,因此形成氢联结。这给水带来了独特的特性,例如相对较高的沸点、高特有热量、凝聚力、粘合性和密度。
Review
::回顾-
Describe the structure of a water molecule.
::描述水分子的结构。 -
What is polarity, and why is water polar?
::什么是极,为什么是水极? -
Explain how hydrogen bonds cause molecules of liquid water to stick together.
::解释氢联结如何导致液态水分子凝聚在一起。 -
What is capillary action? Give an example.
::什么是毛虫作用?举个例子。 -
What property of water helps to maintain homeostasis and how?
::哪些水产有助于保持自足以及如何保持自足?
-
Water molecules are polar, so they form hydrogen bonds. This gives water unique properties, such as a relatively high boiling point, high specific heat, cohesion, adhesion and density.