电
章节大纲
-
What causes a power outage?
::是什么造成停电?In a power outage, all your electrical equipment suddenly stops working. The radio was on just a minute ago and now it is silent. What happened? Somewhere between a power generator and your electrical device was an interruption. Power stopped flowing through the wires and into your radio. That “power” turns out to be electrons that move through the wires and cause an electrical current to flow.
::在停电时,你所有的电动设备都突然停止工作。 无线电在一分钟前就停电了, 现在却停电了。 发生了什么? 电动发电机和你的电动装置之间的某处是中断的。 电力停止了通过电线和无线电的流通。 “ 电源”其实是电源通过电线移动并造成电流流动的电子。Is There Anything Inside an Atom?
::原子里面有东西吗?As the nineteenth century began to draw to a close, the concept of atoms was well-established. We could determine the mass of different atoms and had some good ideas about the atomic composition of many compounds. held that atoms were indivisible, so scientists did not ask questions about what was inside the – it was solid and could not be broken down further. But then things began to change.
::当十九世纪开始接近尾声时,原子的概念早已确立。 我们可以确定不同原子的数量,并且对许多化合物的原子构成有着一些良好的想法。 认为原子是不可分割的,因此科学家们没有询问原子内涵是什么 — — 它是坚固的,无法进一步分解。 但是,事情开始发生变化了。The Electron
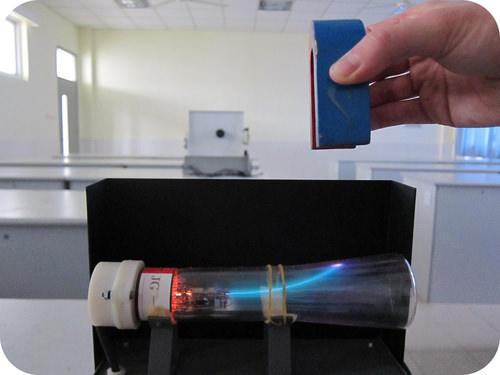
::电子In 1897, English physicist J.J. Thomson (1856-1940) experimented with a device called a , in which an electric current was passed through at low pressure . A cathode ray tube consists of a sealed glass tube fitted at both ends with metal disks called electrodes. The electrodes are then connected to a source of electricity. One electrode , called the anode , becomes positively charged while the other electrode, called the cathode , becomes negatively charged. A glowing beam (the cathode ray) travels from the cathode to the anode.
::1897年,英国物理学家J.J.Thomson(1856-1940年)实验了一个叫做“电流在低压下通过”的装置,阴极射线管由两端安装的密封玻璃管组成,两端都装有金属磁盘,称为电极,然后电极连接到电源。一个称为“阳极”的电极被正电,而另一个称为“阴极”的电极则被负电。一个光束(阴极射线)从阴极到阳极。Earlier investigations by Sir William Crookes and others had been carried out to determine the nature of the cathode ray . Thomson modified and extended these experiments in an effort to learn about these mysterious rays. He discovered two things, which supported the that the cathode ray consisted of a stream of particles.
::威廉·克罗克斯爵士和其他人早些时候进行了调查,以确定阴极射线的性质。汤姆森修改并扩大了这些实验,以了解这些神秘射线。他发现了两件事,支持阴极射线由一串粒子组成。-
When an object was placed between the cathode and the opposite end of the tube, it cast a shadow on the glass.
::当一个物体被放置在阴极和管的对面之间时,它将影子投在玻璃上。 -
A cathode ray tube was constructed with a small metal rail between the two electrodes. Attached to the rail was a paddle wheel capable of rotating along the rail. Upon starting up the cathode ray tube, the wheel rotated from the cathode towards the anode. This proved that the cathode ray was made of particles which must have mass. Crooke had first observed this phenomenon and attributed it to pressure by these particles on the wheel. Thomson correctly surmised that these particles were producing
, which caused the wheel to turn.
::在两个电极之间用小铁轨建造了阴极射线管。 与铁路相连的是能够沿铁路旋转的桨轮。 在启动阴极射线管时, 车轮从阴极射线管旋转到阳极。 这证明阴极射线是由一定质量的粒子组成的。 克罗克首先观察到了这种现象, 并归因于这些粒子对车轮的压力。 汤姆森正确地推测这些粒子正在产生, 从而导致车轮转动。
In order to determine if the cathode ray consisted of charged particles, Thomson used magnets and charged plates to deflect the cathode ray. He observed that cathode rays were deflected by a magnetic field in the same manner as a wire carrying an electric current, which was known to be negatively charged. In addition, the cathode ray was deflected away from a negatively charged metal plate and towards a positively charged plate.
::为了确定阴极射线是否由充电颗粒组成,汤姆森用磁铁和充电板使阴极射线偏向阴极射线,他注意到阴极射线与电流电流电线被磁场以同样方式偏向,已知电流电线被负电,此外,阴极射线从负电荷金属板向正电磁板偏向。Thomson knew that opposite charges attract one another, while like charges repel one another. Together, the results of the cathode ray tube experiments showed that cathode rays are actually streams of tiny negatively charged particles moving at very high speeds. While Thomson originally called these particles corpuscles, they were later named electrons.
::汤姆森知道相反的电荷会相互吸引,而就像电荷互相反射一样。 阴极射线管实验的结果共同表明阴极射线实际上是以非常高的速度移动的微小负电荷粒子流。 尽管汤姆森最初称这些粒子细胞为“粒子细胞 ” , 但它们后来被命名为“电子 ” 。Thomson conducted further experiments, which allowed him to calculate the charge-to-mass ratio of the electron. In units of coulombs to grams, this value is 1.8 × 10 8 Coulombs/gram. He found that this value was a constant and did not depend on the gas used in the cathode ray tube or on the metal used as the electrodes. He concluded that electrons were negatively charged subatomic particles present in atoms of all .
::Thomson进行了进一步实验,从而能够计算电子的电荷-质量比(eme),在千克至千克的单位中,这一数值为1.8 x 108 Coulombs/克。他发现这一数值是一个常数,并不取决于阴极射线管或电极所用金属所使用的气体。他的结论是,电子是所有原子中的负电亚原子颗粒。Summary
::摘要-
Cathode rays are deflected by a magnetic field.
::阴极射线被磁场偏转。 -
The rays are deflected away from a negatively charged electrical field and toward a positively charge field.
::射线从负电磁场向正电磁场偏移。 -
The charge/mass ratio for the electron is 1.8 × 10
8
Coulombs/gram which is equivalent to 1.8 × 10
11
C/kg.
::电子的电荷/重量比率为1.8×108库伦/克,相当于1.8×1011 C/kg。
Review
::回顾-
What subatomic particle creates electric power, and how does it do it?
::什么亚原子粒子能产生电力,它是如何运作的? -
Whose work did Thomson repeat and revise?
::汤姆森重复和修改谁的工作? -
What experiment did Thomson perform that showed cathode rays to be particles?
::汤姆森做了什么实验 显示阴极射线是粒子? -
How did he show that these particles had a charge on them?
::他如何显示这些粒子有电荷? -
Did the cathode ray have positive or negative charge?
::阴极射线有正或负电荷吗?
-
When an object was placed between the cathode and the opposite end of the tube, it cast a shadow on the glass.