Alkaline 地球金属
章节大纲
-
How are oyster shells and chemistry related?
::牡蛎壳和化学如何相关?We take a lot of chemistry for granted. Very few of us think about the chemistry of bone or oyster shells. Both of these materials have large amounts of calcium compounds in them and play important roles in maintaining the structure of the organism. The shell provides a solid surrounding for the oyster. Bones give support to the body so the person can move around and not just be a soft mass of tissue.
::我们把很多化学成分看成是理所当然的。很少人会想到骨头或牡蛎壳的化学成分。这两种材料都含有大量的钙化合物,在维持有机体结构方面起着重要作用。壳为牡蛎提供了坚固的周围。骨骼支持身体,使人体能够移动,而不仅仅是软组织。Alkaline Earth Metals
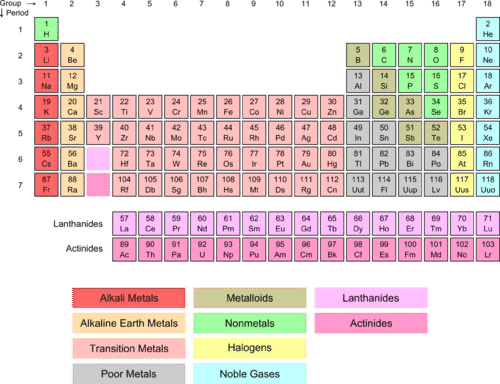
::Alkaline 地球金属Group 2 are referred to as “ alkaline earth ” (tan column below). The name “ alkaline ” comes from the fact that compounds of these elements form basic ( greater than 7) or alkaline solutions when dissolved in water. If the Group 1 elements all have one in their outer , we can predict that the Group 2 elements will have two electrons in that outer shell.
::第2组称为“碱性地球”(以下第10栏),“碱性”是指这些元素的化合物在水溶解时构成基本(大于7)或碱性溶液,如果第1组元素在其外壳中都有一个,我们可以预测第2组元素在该外壳中将有两个电子。The beryllium , the first element of Group 2, has an of four. The atom has the 1 s shell filled as well as the 2 s shell, giving a total of four electrons (1 s 2 2 s 2 ). Note that there are two electrons in the outer shell, a structure that is characteristic of the Group 2 elements. Barium (atomic number 56) has the same outer shell structure of two electrons in the s orbital, even though the internal electron structure for barium is quite complicated.
::第2组中的第一个元素——,即第2组中的第一个元素,有四个。原子填充的是1个贝壳和2个贝壳,共装有4个电子(1s22s2)。请注意,外壳中有两个电子,这是第2组元素的特征。Bior(原子编号56)在轨道上有着与2个电子相同的外壳结构,尽管的内部电子结构相当复杂。Radium (atomic number 88) has similar properties to barium and is also in the Group 2 category. However, radium is a radioactive element and is generally under the category of in addition to being an alkaline earth metal, because it is not a stable element.
:原子号88)与具有相似的特性,也属于第2类,但是,放射性是放射性元素,一般除了是碱性土金属外,还属于放射性元素,因为它不是一个稳定的元素。
The Group 2 elements tend to be less reactive than their Group 1 counterparts. The need to remove two electrons in order for the material to react means more energy is needed for electron removal. However, these elements are reactive enough that they do not exist in their elemental forms in nature, but are present as compounds.
::第2组各单元的反应能力往往低于第1组各单元的反应能力,需要去除两个电子,以便材料作出反应,这意味着电子清除需要更多能量,但是,这些单元的反应能力足以使其在性质上不存在元素形式,而是作为化合物存在。Uses of Alkaline Earth Compounds
::利用阿尔卡林地球化合物Since magnesium burns brightly, it is used in flares and fireworks. Magnesium with aluminum provide light weight and sturdy materials for airplanes, missiles, and rockets. Several antacids use magnesium hydroxide to neutralize excess stomach .
::由于镁燃烧光亮,它被用于照明弹和烟火中,含铝的镁为飞机、导弹和火箭提供了轻重和坚固材料,一些蚂蚁使用氢氧化镁来消除过量的胃部。Calcium compounds are widely found in limestone, marble, and chalk. Calcium is an important constituent of cement. Other uses include calcium chloride as a deicer and limestone as a white pigment in paints and toothpaste.
::钙化合物广泛存在于石灰石、大理石和粉笔中,钙是水泥的重要成分,其他用途包括作为鹿的氯化钙和作为油漆和牙膏中的白色石灰石。Strontium is widely used in fireworks and magnets. Barium compounds can be used in paints, filler for rubber, plastic, and resins, and as a contrast medium for X-rays. Many beryllium compounds are toxic, but these materials have been employed in metal alloys.
::广泛用于烟火和磁铁,化合物可用于油漆、橡胶、塑料和树脂的填料以及X光的对比介质,许多化合物有毒,但这些材料被用于金属合金。Summary
::摘要-
The alkaline earth elements are in Group 2 of the periodic table.
::碱性土元素列于周期表第2组。 -
These elements each have two
s
electrons in their outer shell.
::这些元素的外壳内分别有两个电子。 -
The alkaline earth elements are less reactive than the alkali metals.
::碱性土元素的反应能力低于碱性金属。
Review
::回顾-
Why are these elements known as “alkaline earth” elements?
::为什么这些要素被称为“碱地”要素? -
How many electrons are in the outer shell of the alkaline earth elements?
::碱性土元素外壳内有多少电子? -
Are the alkaline earth elements more or less reactive than the alkali metals? Explain your answer.
::碱性土元素是否比碱性金属或多或少具有反应性? 请解释您的答复 。 -
Is radium usually considered as part of the alkaline earth category in terms of chemistry? Explain your answer.
::从化学角度来说,放射性通常被视为碱性地球类别的一部分吗?解释一下你的答复。
-
The alkaline earth elements are in Group 2 of the periodic table.