协调编号
章节大纲
-
What makes the colors different?
::是什么使颜色不同?The two cobalt salts pictured above both contain Co 2+ . The difference in color is due to the species surrounding the cobalt . The presence of water molecules in the coordination sphere around the central cobalt ion changes the distances among species and the color of the material.
::上方的两座钴盐都含有CO2+。颜色差异是由于钴周围的物种所致。 围绕中央钴离子的协调领域存在水分子,改变物种之间的距离和物质的颜色。Coordination Number
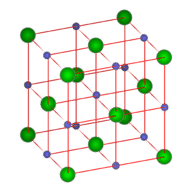
::协调编号The coordination number is the number of ions that immediately surround an ion of the opposite charge within a crystal lattice. If you examine the figure below, you will see that there are six chloride ions immediately surrounding a single sodium ion. The coordination number of sodium is 6. Likewise, six sodium ions immediately surround each chloride ions, making the coordination number of chloride also equal to 6. Because the formula unit of sodium chloride displays a 1:1 ratio between the ions, the coordination numbers must be the same.
::协调号是紧紧围绕晶体层内反电荷离子的离子数。 如果您检查下图, 就会发现在单钠离子周围有6个氯化离子。 钠的协调号是6。 同样, 每氯化离子周围有6个钠离子, 使氯化物的协调号也等于6。 因为氯化钠的公式单位显示离子之间的比例为1: 1, 协调号必须相同 。Lattice structure for sodium chloride. The blue balls represent the sodium ions and the green balls represent the chloride ions
::蓝球代表钠离子,绿球代表氯化离子。The formula unit for cesium chloride is CsCl, also a 1:1 ratio. However, as shown in the figure below, the coordination numbers are not 6 as in NaCl. The center ion is the Cs + ion and is surrounded by the eight Cl − ions at the corners of the cube. Each Cl − ion is also surrounded by eight Cs + ions. The coordination numbers in this type of crystal are both 8. CsCl and NaCl do not adopt identical crystal packing arrangements because the Cs + ion is considerably larger than the Na + ion.
::氯化钙的公式单位为 CsCl, 也是1:1比率。 但是,如下图所示, 协调号不是 NaCl 中的6。 中心离子是 Cs+离子, 周围是立方体角的8 Cl- 离子。 每个 Cl- 离子也周围是 8 Cs+ 离子。 这种晶体的协调号是 8. CsCl 和 NaCl 不采用相同的晶体包装安排, 因为 Cs+离子比 Na+离子大得多。In a cesium chloride crystal, the cesium ion (orange) occupies the center, while the chloride ions (green) occupy each corner of the cube. The coordination number for both ions is 8.
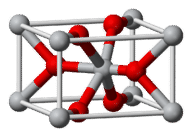
::在氯化镉晶体中,离子(环)占据中心,氯化离子(绿)占据立方体的每个角。两种离子的协调号为8。Another type of crystal is illustrated by titanium(IV) oxide, TiO 2 , which is commonly known as rutile. The rutile crystal is shown below.
::另一种类型的晶体由氧化钛(IV)氧化物(TiO2)加以说明,后者通常称为色质。Titanium(IV) oxide forms tetragonal crystals. The coordination number of the Ti 4+ ions (gray) is 6, while the coordination number of the O 2 − ions (red) is 3.
::Ti4+离子( gray) 的协调号为 6, 而O2- 离子( red) 的协调号为 3。The gray Ti 4+ ions are surrounded by six red O 2− ions. The O 2− ions are surrounded by three Ti 4+ ions. The coordination of the titanium(IV) cation is 6, which is twice the coordination number of the oxide , which is 3. This fits with the formula unit of TiO 2 , since there are twice as many O 2− ions as Ti 4+ ions.
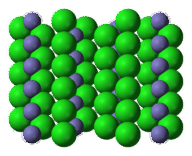
::灰色 Ti4+ 离子周围有 6 个红色 O2 - 离子。 O2 - 离子周围有 3 个 Ti4+ 离子。 钛( IV) 离子的协调为 6 个, 是氧化物的协调数的两倍, 是 3 个 。 这符合 TiO 2 的公式单位, 因为O2 - 离子是 Ti4 + 离子的两倍 。The crystal structure of all must reflect the formula unit. In a crystal of iron(III) chloride, FeCl 3 , there are three times as many chloride ions as iron(III) ions.
::所有的晶体结构必须反映公式单位。在氯化铁(III)的晶体Fecl3中,氯化铁离子是铁(III)离子的三倍。Iron(III) chloride. The bluish-gray Fe 3+ ions are surrounded by green Cl - ions.
::氯化铁(III) 氯化铁。 bluish- gray Fe3+ 离子周围环绕着绿色的氯离子。Summary
::摘要-
The coordination number of a compound is determined by the type and number of ions or other species surrounding a central ion.
::化合物的协调编号由围绕中心离子的离子或其他物种的类型和数量决定。 -
Often the color of a compound is affected by the specific materials coordinated to that central ion.
::化合物的颜色往往受到与中央离子相协调的具体材料的影响。
Review
::回顾-
What is the coordination number for Na
+
in NaCl?
::Na+在NaCl的协调号是多少? -
What is the coordination number for Cs
+
?
::Cs+的协调编号是多少? -
Why are the packing arrangements for Na
+
and Cs
+
different?
::为什么Na+和Cs+的包装安排不同?
-
The coordination number of a compound is determined by the type and number of ions or other species surrounding a central ion.