单一共价债券
章节大纲
-
What holds molecules together?
::什么把分子放在一起?In one form or another, the idea of atoms connecting to form larger substances has been with us for a long time. The Greek philosopher Democritus (460-370 BC) believed that atoms had hooks on them that allowed atoms to connect with one another. Today we believe that atoms are held together by bonds formed when two atoms share a set of electrons, a much more complicated picture than the simple hooks that Democritus preferred.
::在这种或那种形式上,原子与形成较大物质联系的想法已经存在了很长时间了。 希腊哲学哲学家民主党(BC 460-370 ) 认为原子与原子有钩子,让原子相互连接。 今天,我们相信原子由两个原子共享一组电子时形成的债券联在一起,这比民主偏爱的简单钩子复杂得多。Single Covalent Bonds
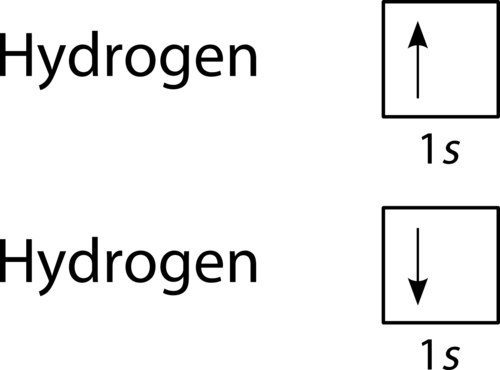
::单一共价债券A forms when two with one each overlap each other. For the hydrogen molecule, this can be shown as:
::一个表单,当两个两个互相重叠时。对于氢分子,这个表单可以显示为:Upon formation of the H 2 molecule, the shared electrons must have opposite spin, so they are shown with opposite spin in the atomic 1 s orbital.
::当H2分子形成时,共享电子必须具有相反的旋转,因此在原子1轨道上以相反的旋转显示。The also form single covalent bonds in their diatomic molecules. An of any halogen , such as fluorine, has seven . Its unpaired electron is located in the 2 p orbital.
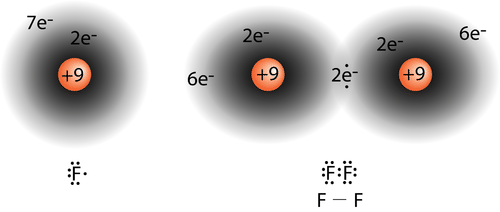
::其二亚原子分子中的单共价键。任何卤素,如氟,都有7个。其未受控电子位于2p轨道上。The single electrons in the third 2 p orbital combine to form the covalent bond:
::第三2p轨道上的单电子结合形成共价结合:On the left is a fluorine atom with seven valence electrons. On the right is the F 2 molecule.
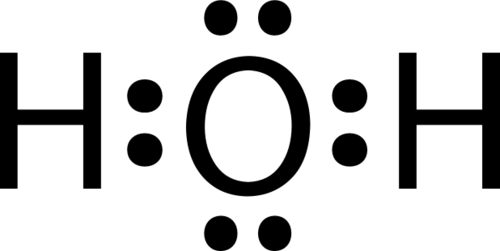
::左边是一个氟原子,有七种等值电子。右边是F2分子。The diatomic fluorine molecule (F 2 ) contains a single shared pair of electrons. Each F atom also has three pair of electrons that are not shared with the other atom. A lone pair is a pair of electrons in a Lewis electron-dot structure that is not shared between atoms. The oxygen atom in the water molecule shown below has two lone pair sets of electrons. Each F atom has three lone pairs. Combined with the two electrons in the covalent bond, each F atom follows the .
::二原子氟化分子(F2)包含单一的共享电子对。每个F原子也拥有三对与另一原子不共享的电子。单对是一对在原子之间不共享的刘易斯电子点结构中的一对电子。以下显示的水分子中的氧原子有两组单对电子。每个F原子有三对单对。结合共价连接中的两对电子,每个F原子都跟随共价连接。Sample Problem 9.1: Lewis Electron Dot Structures
::样本问题9.1:9.1:刘易斯电子点结构Draw the Lewis electron dot structure for water.
::为水绘制刘易斯电子点结构。Step 1: List the known quantities and plan the problem.
::第1步:列出已知数量并规划问题。Known
::已知已知-
of water = H
2
O
::=H2O -
1 O atom = 6 valence electrons
::1 O 原子= 6 等值电子 -
2 H atoms = 2 × 1 = 2 valence electrons
::2 H 原子 = 2 × 1 = 2 等值电子 -
total number of valence electrons = 8
::价值电子总数=8
Use the periodic table to determine the number of valence electrons for each atom and the total number of valence electrons. Arrange the atoms and distribute the electrons so that each atom follows the octet rule. The oxygen atom will have 8 electrons, while the hydrogen atoms will each have 2.
::使用周期表来确定每个原子的值电子数量和值电子的总数。 排列原子并分配电子, 以使每个原子都遵循八点规则。 氧原子将拥有8个电子, 而氢原子将各拥有2个。Step 2: Solve.
::步骤2:解决。for each atom are:
::每个原子的原子为:Each hydrogen atom with its single electron will form a covalent bond with the oxygen atom where it has a single electron. The resulting Lewis electron dot structure is:
::每个氢原子及其单一电子将形成与氧原子的共价联系,在氧原子中,它有一个单一电子。Step 3: Think about your result.
::步骤3:想想你的结果。The oxygen atom follows the octet rule with two pairs of bonding electrons and two lone pairs. Each hydrogen atom follows the octet rule with one bonding pair of electrons.
::氧原子遵循奥克特规则,使用两对联结电子和两对单人电子。每个氢原子都遵循奥克特规则,使用一对联结电子。Summary
::摘要-
Covalent bonds form when electrons in two atoms form overlapping orbitals.
::当两个原子中的电子形成重叠轨道时形成共值键。 -
Lone pair electrons in an atom are not shared with another atom.
::原子中的单对电子不与其他原子共享。
Review
::回顾-
How does a covalent bond form?
::共价债券如何形成? -
What relationship do the spins of shared electrons have with each other?
::共享电子的交汇之间有什么关系? -
Do lone pair electrons form covalent bonds?
::单对电子是否形成共价债券?
-
of water = H
2
O