水结构结构
章节大纲
-
How much of the Earth's surface is covered with water?
::地球表面有多少水覆盖?In his well-known poem “The Rime of the Ancient Mariner,” Samuel Coleridge wrote “Water, water everywhere, Nor any drop to drink.” The narrator was talking about being out on the ocean, but not having any water because he had killed an albatross (apparently bringing bad luck to everyone on the ship). About 75% of the Earth’s surface is water. The major constituent of the human body (over 60%) is water. This simple molecule plays important roles in all kinds of processes.
::塞缪尔·科尔里奇在其著名的诗《古代海军陆战队员的名言 》 中写道 : “ 水 、 水 、 水 、 水 、 水 、 水 、 水 、 水 。 ” 旁白者说要出海,但没有水,因为他杀死了信天翁(显然给船上的每一个人带来厄运 ) 。 地球表面的大约75%是水。 人体的主要成分(超过60 % ) 是水。 这种简单的分子在各种过程中都扮演着重要角色。Structure of Water
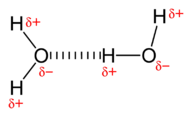
::水结构结构Water is a simple molecule consisting of one oxygen bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). The oxygen atom attracts the shared electrons of the to a significantly greater extent than the hydrogen atoms. As a result, the oxygen atom acquires a partial negative charge , while the hydrogen atoms each acquire a partial positive charge . The molecule adopts a bent structure because of the two lone pairs of electrons on the oxygen atom. The H-O-H bond angle is about 105°, slightly smaller than the ideal 109.5° of an sp 3 hybridized atomic .
::水是一种简单的分子,由一种氧与两种不同的氢原子结合而成。由于氧原子的电子密度较高,这些联结是极共价(极值联结)。氧原子吸引的共享电子比氢原子大得多。因此,氧原子获得部分负电荷(),氢原子各获得部分正电荷()。分子通过一个弯曲结构,因为氧原子上存在两对单电子。H-O-H联结角约为105°,略小于螺旋3混合原子的理想109.5°。The water molecule, visualized three different ways: ball-and-stick model, space-filling model, and structural formula with partial charges. The bent shape of the water molecule is critical because the polar O-H bonds do not cancel one another and the molecule as a whole is polar. Figure illustrates the net of the water molecule. The oxygen is the negative end of the molecule, while the area between the hydrogen atoms is the positive end of the molecule.
::水分子的弯曲形状至关重要, 因为极地 O- H 键不会相互抵消, 而整个分子是极分子。 图显示水分子的网。 氧是分子的负端, 而氢原子之间的区域是分子的正端 。Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. attract one another by dipole-dipole forces as the positive end of one molecule is attracted to the negative end of the nearby molecule. In the case of water, the highly polar O-H bonds results in very little density around the hydrogen atoms. Each hydrogen atom is strongly attracted to the lone-pair electrons on an adjacent oxygen atom. These are called and are stronger than conventional dipole-dipole forces.
::当一个分子的正端被吸引到附近分子的负端时,二极力量相互吸引。在水方面,极极O-H介质在氢原子周围的密度极小。每个氢原子都强烈吸引到相邻氧原子上的单层电子。它们被称为并且比常规的二极力量更强大。A hydrogen bond is the attraction between a lone pair of electrons on the oxygen atom of one molecule and the electron-deficient hydrogen atom of a nearby molecule. Because each oxygen atom has two lone pairs, it can make hydrogen bonds to the hydrogen atoms of two separate other molecules. Figure shows the result – an approximately tetrahedral geometry around each oxygen atom consisting of two covalent bonds and two hydrogen bonds.
::因为每个氧原子都有两对单人氧原子,它能将氢与另外两个分离分子的氢原子联系起来。 图显示了结果 — — 每个氧原子周围大约四面形几何,由两个共价债券和两个氢债券组成。As a result of two covalent bonds and two hydrogen bonds, the geometry around each oxygen atom is approximately tetrahedral. Why does water form droplets? Take a peak at this simulation and see if you can figure it out:
::为什么水会形成滴子?在这个模拟中取一个峰值,看看能不能找出答案:Summary
::摘要-
Water is a molecular compound consisting of polar molecules that have a bent shape.
::水是一种分子化合物,由具有弯曲形状的极分子组成。 -
The oxygen atom acquires a partial negative charge while the hydrogen atom acquires a partial positive charge.
::氧原子获得部分负电荷,氢原子获得部分正电荷。
Review
::回顾-
What type of bond exists in a water molecule?
::水分子中存在何种联系? -
Which part of the molecule has a partial positive charge?
::分子的哪个部分有部分阳性电荷? -
Which part of the molecule has a partial negative charge?
::分子的哪个部分有部分负电荷? -
How do water molecules interact with one another?
::水分子如何相互作用?
-
Water is a molecular compound consisting of polar molecules that have a bent shape.