凝聚反应
章节大纲
-
A new way to lubricate machinery?
::新的润滑机械方法?Vegetable oils are being explored for a variety of uses in which they could replace petroleum products. One such application is in the field of lubricants. Every moving part in machinery (such as engine pistons) needs lubrication to decrease friction and prolong the life of the equipment. Petroleum products serve this purpose now, but are not good for . New techniques for making specialized from vegetable oil are being explored that will make the compounds more stable and more useful as lubricants.
::植物油可以替代石油产品,其中一项应用是润滑油,机械的每一个移动部分(如发动机活塞)都需要润滑剂,以减少摩擦,延长设备寿命。石油产品现在为这一目的服务,但不利于......正在探索利用植物油专门生产植物油的新技术,这将使化合物作为润滑油更稳定、更有用。Condensation Reactions
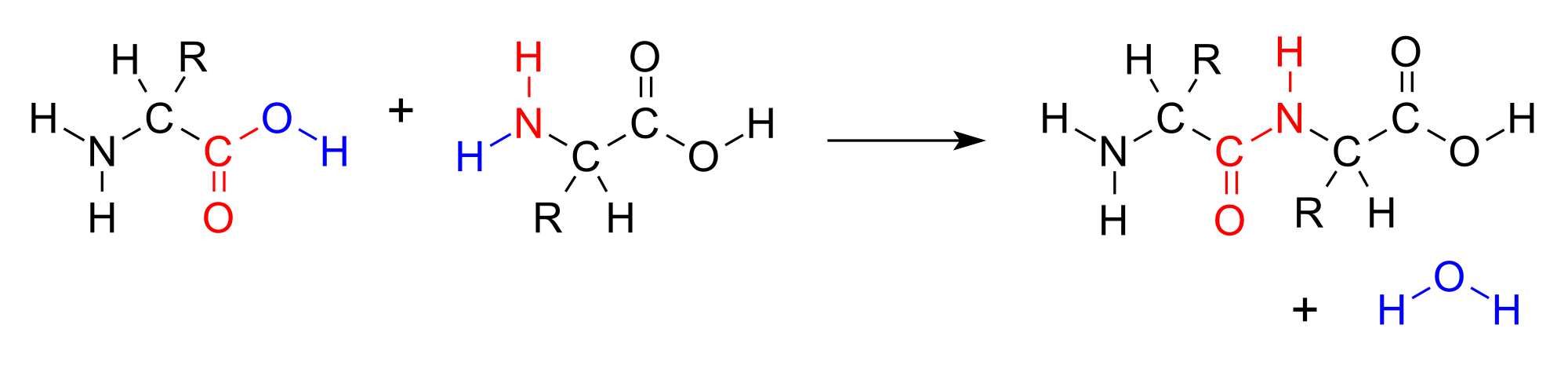
::凝聚反应A condensation reaction is a reaction in which two molecules combine to form a single molecule. A small molecule, often water, is usually removed during a condensation reaction. are important biological molecules that have an functional group on one end of the molecule and a carboxylic acid functional group on the other end. When two amino acids combine in a condensation reaction, a forms between the amine nitrogen of one amino acid and the carboxyl carbon of the second amino acid. A molecule of water is then removed as a second product.
::浓缩反应是一种反应,其中两个分子合并成一个单一分子。一个小分子,通常是水,通常在凝结反应期间被去除。它是重要的生物分子,在分子的一端有一个功能组,在另一端有一个碳酸化物功能组。当两个氨基酸结合成一个凝结反应时,一种是氨基酸的采矿氮,另一种是氨基酸的碳盒。然后,水分子作为第二个产品被删除。Amino acids join together to form a molecule called a dipeptide. The –OH from the carboxyl group of one amino acid combines with a hydrogen atom from the amino group of the other amino acid to produce water (blue).
::氨酸会结合成一种叫做dieptide 的分子。 — — 哦,从一个氨基酸的碳信箱体组与另一个氨基酸(蓝色)的氨基氨基酸组的氢原子结合产生水。This reaction forms a molecule called a dipeptide and the carbon-nitrogen covalent bond is called a peptide bond . When repeated numerous times, a lengthy molecule called a is eventually produced.
::这种反应形成一种叫做dipptide 的分子,而碳-硝基共价联结则被称为peptide联结。当多次重复时,一个称为a的长分子最终会生成。Esterification
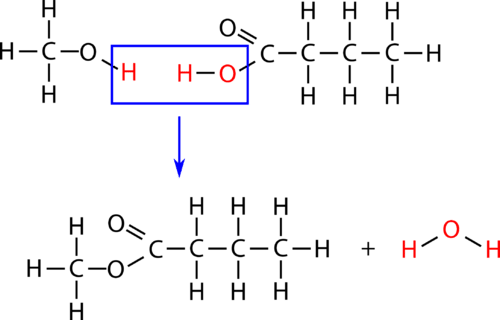
::消化An esterification is a condensation reaction in which an ester is formed from an and a carboxylic acid. Esterification is a subcategory of reactions because a water molecule is produced in the reaction. The reaction is catalyzed by a strong acid , usually sulfuric acid. When the carboxylic acid butanoic acid is heated with an excess of methanol and a few drops of sulfuric acid, the ester methyl butanoate is produced. Methyl butanoate has the scent of pineapples. The reaction is shown below with both molecular and structural formulas .
::酯化是一种凝聚反应,一种酯是由一种碳酸和一种碳酸形成; 消化是一种子反应类别,因为一种水分子是在反应中产生的。 这种反应是用强酸催化的,通常是硫酸。 当箱酸丁亚酸加热时, 产生过量的甲醇和几滴硫酸, 产生过量的甲基丁酸。 甲基丁酸有菠萝的气味。 反应在下面用分子和结构公式显示。The esterification reaction is reversible. When an ester is heated in the presence of a strong base such as sodium hydroxide, the ester breaks down. The products are an alcohol and the conjugate base of the carboxylic acid as a salt .
::蒸发反应是可逆的,当一个酯在氢氧化钠等坚固的基质下被加热时,该酯就会破裂,产品是酒精,是盒状酸作为盐的同质基。
::CH3COOCH2CH3+NaOHCH3COO-NA+CH3CH2OH 乙酸乙烷乙醇乙酸乙酯乙醇乙酯乙酸乙酯乙醇乙酯乙醇乙酯乙醇乙酸乙酸乙酯The sodium hydroxide is not acting as a , but is consumed in the reaction.
::氢氧化钠不是作为一种,而是在反应中消耗。Saponification describes the alkaline hydrolysis reaction of an ester. The term saponification originally described the hydrolysis of long-chain esters called fatty acid esters to produce soap molecules, which are the salts of fatty acids. One such soap molecule is sodium stearate, formed from the hydrolysis of ethyl stearate.
::盐酸化是指一个酯的碱性水解反应,“盐化”一词最初描述了长链酯的水解,称为脂肪酸酯,用于产生肥皂分子,即脂肪酸盐,一种这种肥皂分子是蒸酸钠,由乙酸蒸酸盐的水解形成。
::C17H35 COOC2H5+NaOHC17H35COO-Na+C2H5H乙酸盐Review
::回顾-
What is a condensation reaction?
::什么是凝聚反应? -
What are the starting materials for an esterification reaction?
::产生催化反应的起始材料是什么? -
How can an ester be saponified?
::怎样才能让一个子弟得到宽恕呢?
-
What is a condensation reaction?