氢和阿尔卡利金属
章节大纲
-
You probably think of water as a substance that can put out fires. But some are so reactive that they burn in water. In fact, they virtually explode in water. That’s what is happening in the photo above. About 3 pounds of sodium were added to water, and the result was this explosive reaction. Why is sodium such a reactive element? In this lesson you will find out.
::你可能认为水是一种可以扑灭大火的物质。但有些人反应性很强,以至于在水中燃烧。事实上,它们实际上在水中爆炸。上面的照片就是这样。在水中添加了大约3磅钠,结果就是这种爆炸反应。为什么钠是这样的反应元素?在这个教训中你会发现。The First Group
::第一集团 第一集团Sodium (Na) is an element in group 1 of the periodic table of the elements. This group (column) of the table is shown in Figure . It includes the hydrogen (H) and six that are called alkali metals . Elements in the same group of the periodic table have the same number of . These are the electrons in their outer that can be involved in . Valence electrons determine many of the properties of an element, so elements in the same group have similar properties. All the elements in group 1 have just one valence . This makes them very reactive.
::钠( Na) 是元素周期表第1组中的元素。该组( 列) 显示于图 中。 它包括氢( H) 和六类, 称为碱金属 。 同一组的元素数量相同 。 这些元素是其外表中的电子 。 Valence econens 确定元素的许多属性, 因此同一组的元素具有相似的属性 。 第一组的所有元素只有一种值 。 这使得它们反应性极强 。Q: Why does having just one valence electron make group 1 elements very reactive?
::问:为什么只有一个valence economic 使得第一组元素反应性很强?A: With just one valence electron, group 1 elements are “eager” to lose that . Doing so allows them to achieve a full outer energy level and maximum stability.
::A:只要一个值电子,第1组元素就“渴望”失去它。 这使得它们能够达到完全的外能水平和最大稳定性。Reactivity of Group 1 Elements
::第1组要件的响应性Hydrogen is a very reactive , and the alkali metals are even more reactive. In fact, they are the most reactive metals and, along with the elements in group 17, are the most reactive of all elements. The reactivity of alkali metals increases from the top to the bottom of the group, so lithium (Li) is the least reactive alkali metal and francium (Fr) is the most reactive. Because alkali metals are so reactive, they are found in nature only in combination with other elements. They often combine with group 17 elements, which are very “eager” to gain an electron.
::氢是一种非常反应性的,碱性金属更具有反应性。事实上,它们是最反应性的金属,与第17组中的元素一起,是所有元素中最反应性的。碱性金属从该组的顶部到底部的回作用增加,因此锂(Li)是反应性最小的碱性金属,而(Fr)是最反应性的。由于碱性金属如此反应性,因此在自然中发现它们只与其他元素结合。它们往往与第17组的元素结合,后者非常“渴望”获得电子。Other Properties of Alkali Metals
::Alkali金属的其他属性Besides being very reactive, alkali metals share a number of other properties.
::碱金属除了非常被动反应之外,还具有其他一些特性。-
Alkali metals are all solids at room
.
::阿尔卡利金属都是房间里的固体 -
Alkali metals are low in density, and some of them float on water.
::阿尔卡利金属的密度较低,其中一些金属在水上漂浮。 -
Alkali metals are relatively soft. Some are even soft enough to cut with a knife, like the sodium pictured in the
Figure
.
::阿尔卡利金属相对柔软,有些甚至软到可以用刀切开,如图中描绘的钠。
A Closer Look
::更近看Although all group 1 elements share certain properties, such as being very reactive, they are not alike in every way. Three different group 1 elements are described in more detail below. Notice the ways in which they differ from one another.
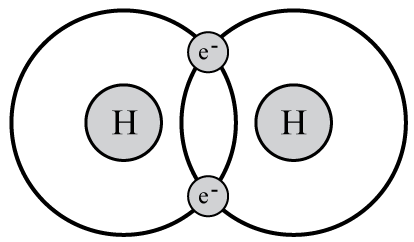
::虽然所有第1组要素都具有某些特性,例如反应性很强,但并非各式各样。下文将更详细地说明第1组三个不同的要素。请注意它们彼此不同的方式。Hydrogen has the smallest, lightest atoms of all elements. Pure hydrogen is a colorless, odorless, tasteless gas that is nontoxic but highly flammable. Hydrogen gas exists mainly as diatomic (“two-atom”) molecules (H 2 ), as shown in the diagram on the right. Hydrogen is the most abundant element in the universe and the third most abundant element on Earth, occurring mainly in compounds such as water.
::纯氢是一种无色、无味、无味、无味的气体,没有毒性,但易燃程度很高,氢气主要作为二原子(“二原子”)分子(H2)存在,如右侧图所示,氢是宇宙中最丰富的元素,是地球上第三最丰富的元素,主要存在于水等化合物中。Q: Why do you think hydrogen gas usually exists as diatomic molecules?
::问题:为什么你认为氢气通常作为二原子分子存在?A: Each hydrogen has just one electron. When two hydrogen atoms bond together, they share a pair of electrons. The shared electrons fill their only energy level, giving them the most stable arrangement of electrons.
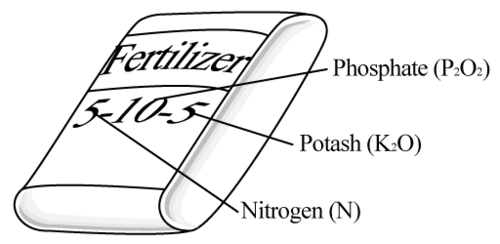
::甲:每一氢只有一个电子。当两个氢原子结合在一起时,它们共享一对电子。共享电子填充着它们唯一的能量水平,给它们最稳定的电子组合。Potassium is a soft, silvery metal that ignites explosively in water. It easily loses its one valence electron to form positive potassium ions (K + ), which are needed by all living cells. Potassium is so important for plants that it is found in almost all fertilizers, like the one shown here. Potassium is abundant in Earth’s crust in minerals such as feldspar.
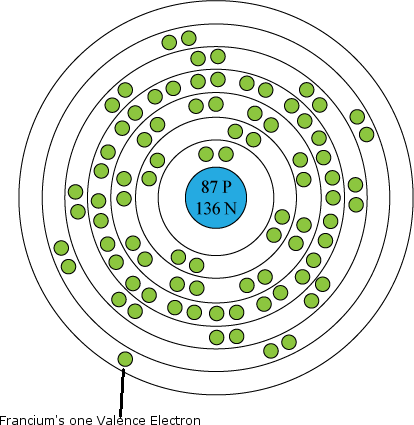
::钾是一种软的银质金属,在水中爆炸性地点燃。 它很容易失去一种能形成所有活细胞都需要的正态钾离子(K+ ) 。 钾对植物非常重要,几乎在所有肥料中都能找到,就像这里所展示的一样。 钾在地球的矿壳中充斥着Feldspar等矿物质。Francium has one of the largest, heaviest atoms of all elements. Its one valence electron is far removed from the nucleus, as you can see in the atomic model on the right, so it is easily removed from the atom. Francium is radioactive and quickly decays to form other elements such as radium. This is why francium is extremely rare in nature. Less than an ounce of francium is present on Earth at any given time.
::是所有元素中最大、最重的原子之一。 它的一个valence econom 离核很远, 正如你从右侧的原子模型中可以看到的, 因此它很容易从原子中移除。 是放射性的, 迅速衰减形成其他元素, 如 。 这就是为什么在自然中极为罕见。 任何时候地球上都存在不到一盎司的。Q: Francium decays too quickly to form compounds with other elements. Which elements to you think it would bond with if it could?
::Q: 的衰变速度太快,无法与其他元素形成化合物。 你认为如果它能够与哪些元素相关联?A: With one valence electron, francium would bond with a halogen element in group 17, which has seven valence electrons and needs one more to fill its outer energy level. Elements in group 17 include fluorine and chlorine.
::A:用一种valence equen, 将与第17组中的卤素元素相联,该组有7种valence eqens, 需要另外一种来填充其外部能量水平, 第17组中的元素包括氟和氯。Summary
::摘要-
Group 1 of the periodic table includes hydrogen and the alkali metals.
::周期表第1组包括氢和碱金属。 -
Because they have just one valence electron, group 1 elements are very reactive. As a result, they are found in nature only in combination with other elements.
::因为它们只有一个valence 电子, 第1组元素反应性很强。 因此,它们只有在与其他元素结合的情况下才能在自然中被发现。 -
Alkali metals are all solids at room temperature. They are relatively soft and low in density.
::碱性金属在室温下都是固体,其密度相对软和低。 -
From the top to the bottom of group 1, the elements have heavier, more reactive atoms.
::从第1组的顶部到底部, 元素有更重, 反应性更强的原子。
Review
::回顾-
What are alkali metals?
::什么是碱性金属? -
Why is hydrogen, a nonmetal, placed in the same group as the alkali metals?
::为什么氢,非金属氢,与碱性金属放在同一组? -
Explain why group 1 elements often form compounds with elements in group 17.
::解释为什么第1组元素往往形成化合物,含有第17组元素。 -
Compare and contrast hydrogen and francium.
::比较和对比氢和。
-
Alkali metals are all solids at room
.