化学等式
章节大纲
-
Look at this rusty bike. It has been left outside in damp weather too many times, so the iron in the metal parts has rusted. Iron rusts when it combines with oxygen in the air. Iron rusting is an example of a . In a chemical reaction, substances change into entirely different substances. For example, the iron in the bike and the oxygen in the air have changed into rust.
::看看这辆生锈的自行车。 它被留在外面的潮湿天气中很多次了, 所以金属部分中的铁已经生锈了。 当它与空气中的氧气结合时, 铁生锈了。 铁生锈就是一个例子。 在化学反应中,物质会变成完全不同的物质。 比如, 自行车中的铁和空气中的氧气已经变成生锈了。Q: How could you represent this reaction, besides just describing it in words?
::问:除了用文字描述,A: Scientists use a standard method to represent a chemical reaction, called a chemical equation .
::A:科学家使用一种标准方法代表一种化学反应,称为化学方程。What Is A Chemical Equation?
::什么是化学等式?A chemical equation is a shorthand way to sum up what occurs in a chemical reaction. The general form of a chemical equation is:
::化学方程式是总结化学反应中发生的情况的一种速记方式。-
-
- Reactants → Products
-
The reactants in a chemical equation are present at the beginning of the reaction, and the products are the substances that are produced in the reaction. The reactants are always written on the left side of the equation and the products on the right. The arrow pointing from left to right shows that the reactants change into the products during the reaction. This happens when break in the reactants and new bonds form in the products. As a result, the products are different chemical substances than the reactants that started the reaction.
::化学方程式中的反应物出现在反应的开头, 产品是反应中产生的物质。 反应物总是写在方程式的左侧和右边的产品上。 箭头从左向右显示反应物在反应时会改变产品。 这发生在反应物断裂和产品中的新债券形成时。 结果, 产品是不同的化学物质, 而不是引发反应的反应物。Q: What is the general equation for the reaction in which iron rusts?
::问题:铁锈反应的一般方程式是什么?A: Iron combines with oxygen to produce rust, which is the named iron oxide. This reaction could be represented by the general chemical equation below. Note that when there is more than one reactant, they are separated by plus signs (+). If more than one product were produced, plus signs would be used between them as well.
::A: 铁与氧结合产生生锈, 也就是所谓的氧化铁。 这种反应可以用下面的一般化学方程式来表示。 请注意, 当有不止一种反应器时, 它们会用加号( +) 来分离。 如果生产了不止一种产品, 它们之间也会使用加号( +) 。-
-
- Iron + Oxygen → Iron Oxide
-
Using Chemical Symbols and Formulas
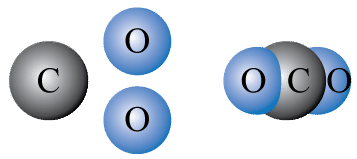
::使用化学符号和公式When scientists write chemical equations, they use chemical symbols and chemical formulas instead of names to represent . Look at the chemical reaction illustrated in the Figure . In this reaction, carbon reacts with oxygen to produce carbon dioxide. Carbon is represented by the chemical symbol C. The chemical symbol for oxygen is O, but pure oxygen exists as diatomic (“two-atom”) molecules, represented by the O 2 . A molecule of the compound carbon dioxide consists of one of carbon and two atoms of oxygen, so carbon dioxide is represented by the chemical formula CO 2 .
::当科学家写化学方程式时,他们使用化学符号和化学方程式来代表 。看看图中显示的化学反应。在这一反应中,碳与氧反应产生二氧化碳。碳以化学符号C为代表。氧的化学符号为O,但纯氧作为二亚原子的分子存在,以O为代表。复合二氧化碳的分子由碳和氧的两个原子组成,因此二氧化碳以化学公式CO2为代表。Q: What is the chemical equation for this reaction?
::问题:这种反应的化学方程式是什么?A: The chemical equation is:
::A:化学方程式是:-
-
- C + O 2 → CO 2
-
Q: How have the atoms of the reactants been rearranged in the products of the reaction? What bonds have been broken, and what new bonds have formed?
::问题:反应产物是如何重新排列反应体原子的?哪些债券被打破,哪些新的债券已经形成?A: Bonds between the oxygen atoms in the oxygen molecule have been broken, and new bonds have formed between the carbon atom and the two oxygen atoms.
::A:氧原子分子中的氧原子的结合已经破裂,碳原子和两个氧原子之间形成了新的联系。Is It Balanced?
::平衡了吗?All chemical equations, like equations in math, must balance. This means that there must be the same number of each type of atom on both sides of the arrow. That’s because matter is always conserved in a chemical reaction. This is the .
::所有化学方程式,就像数学中的方程式一样,都必须平衡。 这意味着箭头两侧的原子数量必须相同。 这是因为物质总是在化学反应中被保存。 这就是问题所在。Look at the equation above for the reaction between carbon and oxygen in the formation of carbon dioxide. Count the number of atoms of each type. Are the numbers the same on both sides of the arrow? The answer is yes, so the equation is balanced.
::查看上面的方程式, 以了解二氧化碳形成过程中碳与氧之间的反应。 计算每类原子的数量。 箭头两侧的数字是否相同? 答案是是是的, 所以方程式是平衡的 。Coefficients
::系数系数Let’s return to the chemical reaction in which iron (Fe) combines with oxygen (O 2 ) to form rust, or iron oxide (Fe 2 O 3 ). The equation for this reaction is:
::让我们回到铁(Fe)与氧(O2)结合形成生锈或氧化铁(Fe2O3)的化学反应,-
-
- 4Fe+ 3O 2 → 2Fe 2 O 3
-
This equation illustrates the use of coefficients to balance chemical equations. A coefficient is a number placed in front of a chemical symbol or formula that shows how many atoms or molecules of the substance are involved in the reaction. From the equation for rusting, you can see that four atoms of iron combine with three molecules of oxygen to form two molecules of iron oxide.
::此方程式显示了平衡化学方程式的系数。 系数是化学符号或公式前面的一个数字, 显示该物质的原子或分子在反应中涉及多少个原子或分子。 从生锈的方程式中可以看出, 四个铁原子与三个氧分子结合, 形成两个氧化铁分子。Q: Is the equation for the rusting reaction balanced? How can you tell?
::问题:生锈反应的方程式平衡吗?你怎么知道?A: Yes, the equation is balanced. You can tell because there is the same number of each type of atom on both sides of the arrow. First count the iron atoms. There are four iron atoms in the reactants. There are also four iron atoms in the products (two in each of the two iron oxide molecules). Now count the oxygen atoms. There are six on each side of the arrow, confirming that the equation is balanced in terms of oxygen as well as iron.
::A: 是的, 方程式是平衡的。 您可以分辨出箭头两侧每种原子的数量相同。 首先计算铁原子。 反应器中共有四个铁原子。 产品中还有四个铁原子( 两个氧化铁分子中各有两个) 。 现在计数氧原子。 箭头两侧各有六个, 证实方程式在氧和铁方面是平衡的。Summary
::摘要-
Scientists use chemical equations to summarize what happens in chemical reactions. Reactants are placed on the left side of the equation and products are placed on the right. An arrow is used to indicate the direction in which the reaction occurs. Plus signs (+) are placed between multiple reactants or products.
::科学家利用化学方程式来总结化学反应中发生的情况。反应剂放在方程式的左侧,产品放在右侧。箭头用来表示反应的方向。加号(+)放在多个反应剂或产品之间。 -
In chemical equations, reactants and products are represented by chemical symbols and formulas. Numbers called coefficients are placed in front of the symbols and formulas to show how much of each substance is involved in the reaction.
::在化学方程式中,反应物和产品由化学符号和公式代表,称为系数的数字放在符号和公式前面,以显示每种物质在反应中涉及多少。 -
Chemical equations must be balanced. A balanced equation has the same number of each type of atom on both sides of the equation.
::化学方程式必须是平衡的。平衡的方程式在方程式两侧的原子类型数量相同。
Review
::回顾-
What is a chemical equation? Identify the parts of a chemical equation.
::什么是化学方程? 识别化学方程的各个部分 。 -
Write a chemical equation for the chemical reaction in which calcium carbonate (CaCO
3
) produces calcium oxide (CaO) and carbon dioxide (CO
2
).
::为碳酸钙(CaCO3)产生氧化钙(CaO)和二氧化碳(CO2)的化学反应写化学方程。 -
Describe in words the chemical reaction represented by the following chemical equation: 2NO
2
→ 2O
2
+ N
2
::用以下化学方程式表示的化学反应用文字描述: 2NO2 2O2 + N2 -
When is a chemical equation balanced?
::化学方程式何时平衡?
Resources
::资源 -