2.17 国家更迭
章节大纲
-
Both of these photos show the famous Golden Gate Bridge near San Francisco, California. The pictures were taken from about the same point of view, but they look very different. In the picture on the left, the deck of the bridge is almost completely hidden by a thick layer of fog. In the picture on the right the fog has disappeared, and the deck of the bridge—as well as the water below it—is clearly visible. Fog consists of tiny droplets of water. The fog in the picture is like a cloud at ground level. Where did the fog come from, and where did it go?
::这两张照片都展示了加州旧金山附近著名的金门大桥。这些照片是从同样的角度拍摄的,但看起来非常不同。在左边的图片中,大桥的甲板几乎完全被浓雾层遮蔽。在右边的图片中,雾已经消失,大桥的甲板和下方的水明显可见。雾是由小滴水构成的。照片中的雾像地面的云一样。雾从何而来,又从何而去呢?What Are Changes of State?
::什么是国家更迭?The water droplets of fog form from water vapor in the air. Fog disappears when the water droplets change back to water vapor. These changes are examples of changes of state. A occurs whenever matter changes from one state to another. Common on Earth are , liquid, and . Matter may change back and forth between any two of these states.
::雾从空气中的水蒸气中形成。 当水滴又变成水蒸气时,雾就会消失。 这些变化是状态变化的例子。 当物质从一个状态变化到另一个状态时, 就会发生。 在地球上常见的是, 液体, 和。 物质可能会在这两个状态中的任何两个状态之间发生反向变化 。Changes of state are physical changes in matter. They are reversible changes that do not change matter’s chemical makeup or chemical properties. For example, when fog changes to water vapor, it is still water and can change back to liquid water again.
::状态变化是物质物质物理变化。 状态变化是不可逆的变化,不会改变物质的化学构成或化学特性。 比如,当雾改变水蒸气时,它仍然是水,并且可以再次变回液态水。Processes that Cause Changes of State
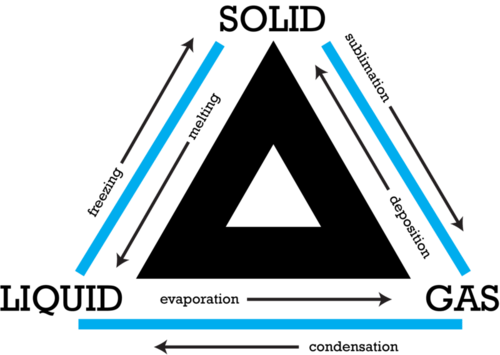
::导致国家更替的程序Several processes are involved in common changes of state. They include , , , , , and . The Figure shows how matter changes in each of these processes.
::一些进程涉及共同的状态变化,包括 、 、 、 、 和 。 图显示每个进程发生物质变化的情况 。Q: Which two processes result in matter changing to the solid state?
::问题:哪些两个过程导致物质转变为固态?A: The processes are deposition, in which matter changes from a gas to a solid, and freezing, in which matter changes from a liquid to a solid.
::A: 过程是沉积,其中物质从气体变成固体,冷冻,其中物质从液体变成固体。The Role of Energy in Changes of State
::能源在改变国家中的作用Suppose that you leave some squares of chocolate candy in the hot sun. A couple of hours later, you notice that the chocolate has turned into a puddle like the one pictured in the Figure .
::假设你把一些方块巧克力糖留在炎热的阳光下。几个小时后,你注意到巧克力已经变成像图中图中的水坑。Q: What happened to the chocolate?
::问:巧克力怎么了?A: The chocolate melted. It changed from a solid to a liquid.
::A:巧克力融化了。它从固体变成液体。In order for solid chocolate to melt and change to a liquid, the particles of chocolate must gain . The chocolate pictured in the Figure gained energy from sunlight. Energy is the ability to cause changes in matter, and it is always involved in changes of state. When matter changes from one state to another, it either absorbs energy—as when chocolate melts—or loses energy. For example, if you were to place the melted chocolate in a refrigerator, it would lose energy to the cold air inside the refrigerator. As a result, the liquid chocolate would change to a solid again.
::为了让固体巧克力融化并变成液体,巧克力的粒子必须增加。图中描绘的巧克力从阳光中获得能量。能量是改变物质的能力,它总是涉及状态的变化。当物质从一个州变化到另一个州时,它要么吸收能量——就像巧克力融化那样——要么失去能量。例如,如果你把融化的巧克力放在冰箱里,它就会失去能量,让冷空气进入冰箱。结果,液体巧克力就会再次变成固体。Q: Why is energy always involved in changes of state?
::问:为什么能源总是卷入国家变化?A: The energy of particles of matter determines the matter's state. Particles of a gas have more energy than particles of a liquid, and particles of a liquid have more energy than particles of a solid. Therefore, in order for matter to change from a solid to a liquid or from a liquid to a gas, particles of matter must absorb energy. In order for matter to change from a gas to a liquid or from a liquid to a solid, particles of matter must lose energy.
::A:物质粒子的能量决定着物质状态。气体粒子的能量比液体粒子的能量多,而液体粒子的能量比固体粒子的能量多。因此,如果物质从固体变成液体,或从液体变成气体,物质粒子必须吸收能量。如果物质物质从气体变成液体,或从液体变成固体,物质粒子必须失去能量。Summary
::摘要-
A change of state occurs whenever matter changes from one state to another. Changes of state are physical changes in matter. They are reversible changes that do not change matter’s chemical makeup or chemical properties.
::国家的变化是物质的物理变化。 国家的变化是可逆的改变,不会改变物质的化学构成或化学特性。 -
Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation.
::国家变化所涉及的过程包括熔化、冻结、升华、沉积、凝固和蒸发。 -
Energy is always involved in changes of state. Particles of matter either absorb or lose energy when matter changes from one state to another.
::能源总是涉及国家的变化,当物质从一个州向另一个州变化时,物质粒子要么吸收,要么失去能源。
Review
::回顾-
Define change of state, and give an example.
::定义状态变化, 并举一个例子 。 -
Identify processes that change matter to a liquid state.
::查明将重要改变为液态的流程。 -
Why must energy be absorbed to change a liquid to a gas?
::为什么必须吸收能源将液体转化为气体?
Resources
::资源 -
A change of state occurs whenever matter changes from one state to another. Changes of state are physical changes in matter. They are reversible changes that do not change matter’s chemical makeup or chemical properties.