4.11 卤素
章节大纲
-
You’ve probably seen halogen lights like the ones pictured here. You may even have halogen lights in your home. If you do, you may have noticed that they get really hot and give off a lot of light for their size. A halogen light differs from a regular incandescent light bulb in having a small amount of halogen inside the bulb. The gas combines chemically with the metal in the filament, and this extends the life of the filament. It allows the lamp to get hotter and give off more light than a regular incandescent light without burning out quickly. What is halogen gas, and which are halogens? In this article, you’ll find out.
::你可能见过像这里所描绘的卤素灯光。 你甚至可能看到家里有卤素灯光。 如果你看到,你可能注意到它们变得非常热,并会照亮很多大小的光。 卤素灯光与普通的白种灯灯灯灯灯泡不同,因为灯泡内有少量卤素。 气体化学地结合了丝质中的金属,延长了丝质的寿命。 它允许灯光变热,发光比正常的白种灯光更亮,而不会迅速燃烧。什么是卤素气,什么是卤素? 在文章中,你会发现什么是卤素? 在文章中,你会发现什么是卤素?Meet the Halogens
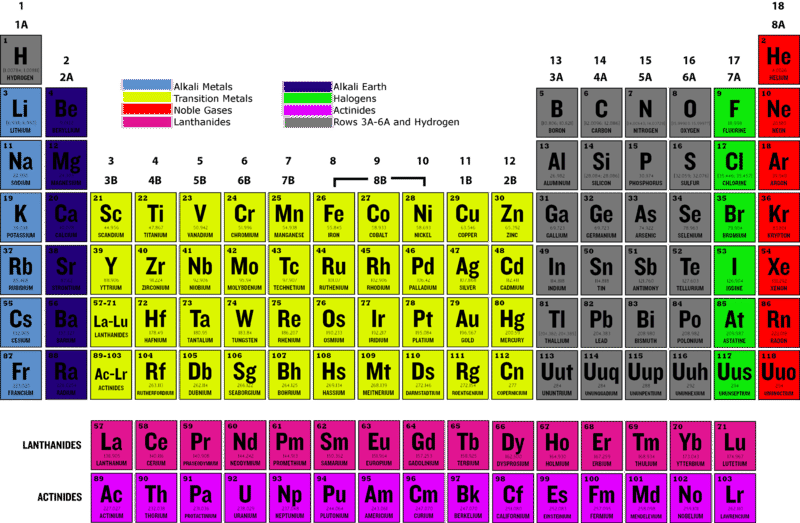
::和Halogens见面Halogens are highly reactive nonmetallic elements in group 17 of the periodic table . As you can see in the periodic table , the halogens include the elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). All of them are relatively common on Earth except for astatine. Astatine is radioactive and rapidly decays to other, more stable elements. As a result, it is one of the least common elements on Earth.
::卤素是周期表第17组中高度反应性的非金属元素。正如您在周期表中看到的那样,卤素包括氟(F)、氯(Cl)、溴(Br)、碘(I)和阿斯特丁(At)等元素。除阿斯塔丁外,这些元素在地球上都相对常见。阿斯塔因是放射性的,迅速衰减到其他更稳定的元素。因此,它是地球上最不常见元素之一。Q: Based on their position in the periodic table from the Figure , how many do you think halogens have?
::问题:根据他们在从图中得出的定期表格中的位置, 你认为卤素有多少?A: The number of valence electrons starts at one for elements in group 1. It then increases by one from left to right across each period (row) of the periodic table for groups 1–2 and 13–18 (numbered 3-0 in the periodic table above.) Therefore, halogens have seven valence electrons.
::A: 第1组各元素的值值电子数从第1组的一个开始,然后在第1至2组和13至18组的周期表的每个周期(行)之间从左向右增加一个(在上文的周期表中为3至0)。 因此,卤素有7个值电子。Chemical Properties of Halogens
::卤素化学特性The halogens are among the most reactive of all elements, although reactivity declines from the top to the bottom of the halogen group. Because all halogens have seven valence electrons, they are “eager” to gain one more . Doing so gives them a full outer , which is the most stable arrangement of electrons. Halogens often combine with alkali metals in group 1 of the periodic table. Alkali metals have just one valence electron, which they are equally “eager” to donate. Reactions involving halogens, especially halogens near the top of the group, may be explosive. You can see some examples in the video below. ( Warning: Don’t try any of these reactions at home!)
::卤素是所有元素中反应最强的元素之一,尽管从卤素组的顶部到底部的回反应性会下降。由于所有卤素都有七种valence 电子,它们“渴望”再获得一个。这样做可以给他们一个完整的外表,这是最稳定的电子组合。卤素往往与周期表第1组的碱性金属结合。 Alkali金属只有一种valence 电子,它们同样是捐赠的“渴望 ” 。 涉及卤素的反应,特别是靠近该组顶部的卤素,可能是爆炸性的。 您可以在下面的视频中看到一些例子。 (警告:不要在家中尝试任何这些反应! )Physical Properties of Halogens
::卤素的物理特性The halogen group is quite diverse. It includes elements that occur in three different at room . Fluorine and chlorine are gases, bromine is a , and iodine and astatine are solids. Halogens also vary in color, as you can see in the Figure . Fluorine and chlorine are green, bromine is red, and iodine and astatine are nearly black. Like other , halogens cannot conduct electricity or . Compared with most other elements, halogens have relatively low and points.
::卤素组是多种多样的,它包括三个不同房间发生的元素。氟和氯是气体,溴是气体,碘和阿斯塔因是固体。卤素在颜色上也不同,如图所示。氟和氯是绿色的,溴是红色的,碘和阿斯特因几乎是黑色的。与其他元素一样,卤素不能进行电力或。与大多数其他元素相比,卤素较低,点数也较低。Uses of Halogens
::卤素用途Most halogens have a variety of important uses. A few are described in the Figure .
::多数卤素有各种重要用途,图中描述了其中几个。Q: Can you relate some of these uses of halogens to the properties of these elements?
::问题:你能否将这些卤素的某些用途与这些元素的特性联系起来?A: The ability of halogens to kill germs and bleach clothes relates to their highly reactive nature.
::A:卤素杀死细菌和漂白衣的能力与其高度反应性有关。Summary
::摘要-
Halogens are highly reactive nonmetal elements in group 17 of the periodic table.
::卤素是周期表第17组中反应性很强的非金属元素。 -
Halogens include solids, liquids, and gases at room temperature, and they vary in color.
::卤素包括固体、液体和室温气体,颜色不同。 -
Halogens are among the most reactive of all elements. They have seven valence electrons, so they are very “eager” to gain one electron to have a full outer energy level.
::卤素是所有元素中最具反应力的元素之一。它们有七种价值电子,因此它们非常“渴望”获得一种电子,以获得完整的外能水平。 -
Halogens have a variety of important uses, such as preventing tooth decay and killing germs.
::卤素有多种重要用途,如防止牙齿腐烂和杀菌。
Review
::回顾-
What are halogens?
::什么是卤素? -
Why are halogens very reactive?
::为什么卤素反应性很强? -
Describe the physical properties of halogens.
::描述卤素的物理特性。 -
Why is chlorine added to swimming pool water?
::为什么将氯添加到游泳池水中?
-
Halogens are highly reactive nonmetal elements in group 17 of the periodic table.