4.12 惰性气体
章节大纲
-
Okay, helium balloons are light, but they’re not that light! This fanciful picture serves to make the point that helium is one of the lightest . Helium belongs to a group of elements called the noble gases.
::好了,氦气球是轻的,但是它们不是光!这幅奇观的图画可以说明氦是最轻的。 属于一组称为惰性气体的元素。What Are Noble Gases?
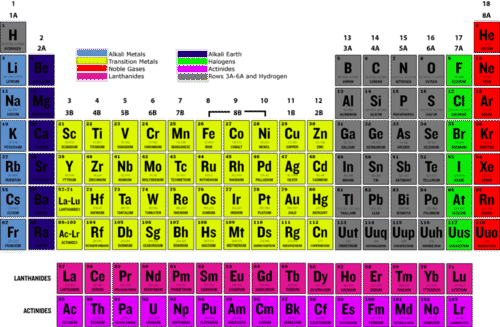
::什么是高贵的气体?Noble gases are nonreactive, nonmetallic elements in group 18 of the periodic table . As you can see in the periodic table below, noble gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). All noble gases are colorless and odorless. They also have low points, explaining why they are at room . Radon, at the bottom of the group, is radioactive, so it constantly decays to other elements.
::惰性气体是周期表第18组中的非反应性、非金属元素。如下表所示,惰性气体包括(He)、奈恩(Ne)、阿贡(Ar)、克赖普顿(Kr)、xenon(Xe)和radon(Rn)。所有惰性气体都是无色和无味的。它们也有低点,解释它们为什么在房间。在组的底部,Radon是放射性的,因此它会不断腐蚀到其他元素中。Q: Based on their position in the periodic table ( Figure ), how many do you think noble gases have?
::问题:根据他们在周期表(图)中的位置, 你认为惰性气体有多少?A: The number of valence electrons starts at one for elements in group 1. It then increases by one from left to right across each period (row) of the periodic table for groups 1–2 and 13–18 (numbered 3–0 in the table above). Therefore, noble gases have eight valence electrons.
::A:第1组元素的值值电子数从第1组元素的1个开始,然后在第1-2组和13-18组周期表的每个周期(行)之间从左向右增加1个(上表为3-0 ) 。 因此,惰性气体有8个值电子。Chemical Properties of Noble Gases
::惰性气体的化学属性Noble gases are the least reactive of all known elements. That’s because with eight valence electrons, their outer levels are full. The only exception is helium, which has just two electrons. But helium also has a full outer , because its only energy level (energy level 1) can hold a maximum of two electrons. A full outer energy level is the most stable arrangement of electrons. As a result, noble gases cannot become more stable by reacting with other elements and gaining or losing valence electrons. Therefore, noble gases are rarely involved in and almost never form compounds with other elements.
::惰性气体是所有已知元素中反应最小的。 这是因为有8种等值电子,它们的外部水平是满的。 唯一的例外是,它只有两个电子。 但也有一个完整的外部,因为它唯一的能量水平(能源水平 1 ) 最多能维持两个电子。 完整的外能水平是电子的最稳定的组合。 结果,惰性气体无法通过与其他元素反应和获得或失去等值电子而变得更加稳定。 因此,惰性气体很少与其他元素混合在一起,也几乎从来不会与其他元素形成化合物。Noble Gases and the Octet Rule
::惰性气体和奥克特规则Because the noble gases are the least reactive of all elements, their eight valence electrons are used as the standard for nonreactivity and to explain how other elements interact. This is stated as the octet (“group of eight”) rule. According to this rule, atoms react to form compounds that allow them to have a group of eight valence electrons like the noble gases. For example, sodium (with one valence electron) reacts with chlorine (with seven valence electrons) to form the stable sodium chloride (table salt). In this reaction, sodium donates an and chlorine accepts it, giving each element an octet of valence electrons.
::由于惰性气体是所有元素中反应最小的,因此它们的八种等值电子被用作无反应的标准,并用来解释其他元素是如何相互作用的。这被称为八点规则(“八组 ”) 。 根据这一规则,原子会反应形成化合物,从而形成八种等惰性气体等八种等值电子。例如,钠(同一种等值电子)与氯(同七种等值电子)发生反应,形成稳定的氯化钠(同盐 ) 。在这种反应中,钠会捐赠一种氯,给每种元素一个等值电子。Some Uses of Noble Gases
::惰性气体的某些用途Did you ever get a birthday balloon like the one pictured ? The balloon is filled with the noble gas helium. The gas is pumped from a tank into a Mylar balloon. Unlike a balloon filled with air, a balloon filled with helium needs to be weighted down so it won’t float away.
::气球充斥着惰性气体氦气。 气球从一个罐子抽到一个麦拉尔气球。 与充气气气球不同的是,一个装满氦气的气球需要加权下来,这样它就不会飘走。Q: Why does a helium balloon float away if it’s not weighted down?
::问:如果不加权下调,为什么一个氦气球会飘走?A: Helium atoms have just two , two , and two electrons, so they have less mass than any other atoms except hydrogen. As a result, helium is lighter than air, explaining why a helium balloon floats up into the air unless weighted down.
::A: 原子只有两个、两个和两个电子,因此它们的质量小于除氢以外的任何其他原子。因此,氦比空气轻,解释了为何除非加权,否则氦气球会浮向空气。Early incandescent light bulbs, like the one pictured in the Figure , didn’t last very long. The filaments quickly burned out. Although air was pumped out of the bulb, it wasn’t a complete vacuum. Oxygen in the small amount of air remaining inside the light bulb reacted with the metal filament. This corroded the filament and caused dark deposits on the glass. Filling a light bulb with argon gas prevents these problems. That’s why modern light bulbs are filled with argon.
::早期的白炽灯泡,如图中描绘的灯泡,没有持续很长时间。 丝状灯泡迅速熄灭。 虽然空气是从灯泡中抽出的,但它不是一个完整的真空。 光灯灯泡中的少量空气中的氧与金属丝状反应。 这腐蚀了丝状,并造成玻璃上的暗层。 装满了芳香气的灯泡,防止了这些问题。 这就是为什么现代灯泡充满了芳香。Q: How does argon prevent the problems of early light bulbs?
::问题:方形如何防止早期灯泡的问题?A: As a noble gas with eight electrons, argon doesn’t react with the metal in the filament. This protects the filament and keeps the glass blub free of deposits.
::A:作为拥有八种电子的惰性气体,argon不会与丝状金属发生反应。 这保护了丝状,并避免了玻璃薄膜的沉积。Noble gases are also used to fill the glass tubes of lighted signs like the one in the Figure . Although noble gases are chemically nonreactive, their electrons can be energized by sending an electric current through them. When this happens, the electrons jump to a higher energy level. When the electrons return to their original energy level, they give off energy as light. Different noble gases give off light of different colors. Neon gives off reddish-orange light, like the word “Open” in the sign below. Krypton gives off violet light and xenon gives off blue light.
::惰性气体也用于填充像图中那样的光标志的玻璃管。 虽然惰性气体在化学上没有反应, 但是它们的电子可以通过电流通过它们而得到动力。 发生这种情况时, 电子会跳到更高的能量水平。 当电子回到原来的能量水平时, 它们会以光的形式释放能量。 不同的惰性气体会发出不同颜色的光。 尼昂会发出红丝- 橙色的光, 就像下面的符号“ 打开” 那样。 Krypton 会发出紫外线灯, xenon 发出蓝色的光 。Summary
::摘要-
Noble gases are nonreactive, nonmetallic elements in group 18 of the periodic table.
::惰性气体是周期表第18组中的非反应性、非金属元素。 -
Noble gases are the least reactive of all elements. That’s because they have eight valence electrons, which fill their outer energy level. This is the most stable arrangement of electrons, so noble gases rarely react with other elements and form compounds.
::惰性气体是所有元素中反应最小的。 这是因为它们有8种价值电子,它们填充其外能水平。 这是最稳定的电子组合,因此惰性气体很少与其他元素和化合物发生反应。 -
The octet rule states that atoms react to form compounds that allow them to have eight valence electrons like the noble gases, which are the least reactive elements.
::octet 规则规定,原子对形成化合物的化合物作出反应,允许它们拥有八种价值电子,如惰性气体,它们是反应最小的元素。 -
Noble gases are used for balloons, light bulbs, and lighted signs.
::惰性气体用于气球、灯泡和照明标志。
Review
::回顾-
What are noble gases?
::什么是惰性气体? -
Explain why noble gases are almost completely nonreactive.
::解释为什么惰性气体几乎完全没有反应。 -
What is the octet rule? How is it related to noble gases?
::octet规则是什么?它与惰性气体有什么关系? -
Hydrogen (H) atoms have one electron and exist as diatomic (“two-atom”) molecules (H
2
). Helium atoms have two electrons and exist only as single helium atoms. Explain why hydrogen and helium differ in this way.
::氢(H)原子有一个电子,作为二原子(“两原子”)分子存在(H2)。 原子有两个电子,仅作为单原子存在。解释为什么氢和氦以这种方式不同。
Resources
::资源 -
Noble gases are nonreactive, nonmetallic elements in group 18 of the periodic table.