5.13 活性剂和产品
章节大纲
-
Did you ever wonder what happens to a candle when it burns? A candle burning is a in matter. In a chemical change, one type of matter changes into a different type of matter, with different chemical properties. Chemical changes occur because of .
::你有没有想过蜡烛烧着时会发生什么事?蜡烛烧着是一个问题。在化学变化中,一种物质会改变为另一种物质,具有不同的化学特性。化学变化会因化学变化而发生。From Reactants to Products
::从反应剂到产品All chemical reactions—including a candle burning—involve reactants and products.
::所有化学反应——包括蜡烛燃烧——都与反应器和产品有关。-
Reactants
are substances that start a chemical reaction.
::活性剂是开始化学反应的物质。 -
Products
are substances that are produced in the reaction.
::产品是反应中产生的物质。
When a candle burns, the reactants are fuel (the candlewick and wax) and oxygen (in the air). The products are carbon dioxide and water vapor.
::当蜡烛燃烧时,反应器是燃料(蜡烛和蜡)和氧(空气中),产品是二氧化碳和水蒸气。Relating Reactants and Products
::相关活性剂和产品The relationship between reactants and products in a chemical reaction can be represented by a chemical equation that has this general form:
::在化学反应中,反应物与产品之间的关系可以用一种化学方程式来表示,化学方程式具有这种一般形式:-
-
- Reactants → Products
-
The arrow (→) shows the direction in which the reaction occurs. In many reactions, the reaction also occurs in the opposite direction. This is represented with another arrow pointing in the opposite direction (←).
::箭头显示反应发生的方向。 在许多反应中, 反应也发生在相反的方向。 这是用另一个箭头表示的, 指向相反的方向 () 。
Q: Write a general chemical equation for the reaction that occurs when a fuel such as candle wax burns.
::问题:为蜡烛燃烧等燃料产生的反应写出一般化学方程式。A: The burning of fuel is a . The general equation for this type of reaction is:
::A:燃料燃烧是一个.这种反应的一般方程式是:-
-
- Fuel + O 2 → CO 2 + H 2 O
-
Q: How do the reactants in a chemical reaction turn into the products?
::问题:化学反应中的反应剂如何变成产品?A: Bonds break in the reactants, and new bonds form in the products.
::A: 反应者的债券破裂,产品中出现新的债券形式。Breaking and Making Chemical Bonds
::打破和制造化学债券The reactants and products in a chemical reaction contain the same atoms, but they are rearranged during the reaction. As a result, the atoms end up in different combinations in the products. This makes the products new substances that are chemically different from the reactants.
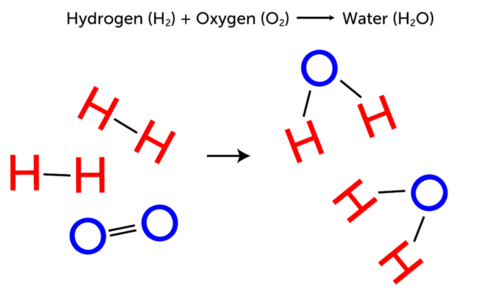
::化学反应中的反应物和产品含有相同的原子,但在反应期间会重新排列,因此原子最终在产品中形成不同的组合,从而使产品中的新物质在化学上与反应物不同。Consider the example of water forming from hydrogen and oxygen. Both hydrogen and oxygen gases exist as diatomic (“two-atom”) molecules. These molecules are the reactants in the reaction. The Figure shows that bonds must break to separate the atoms in the hydrogen and oxygen molecules. Then new bonds must form between hydrogen and oxygen atoms to form water molecules. The water molecules are the products of the reaction.
::以氢和氧形成水的例子为例。氢和氧的气体都作为二原子分子存在。这些分子是反应中的反应物。图中显示,介质必须断裂以分离氢和氧分子中的原子。然后,氢和氧原子之间必须形成新的联结以形成水分子。水分子是反应的产物。Q: Watch the animation of a similar chemical reaction at the following URL. Can you identify the reactants and the product in the reaction?
::问题:在下面的 URL 上观察类似化学反应的动画。您能识别反应中的反应器和产品吗?A: The reactants are hydrogen (H 2 ) and fluorine (F 2 ), and the product is hydrogen fluoride (HF).
::A:反应剂为氢(H2)和氟(F2),产品为氟化氢(HF)。Summary
::摘要-
All chemical reactions involve both reactants and products. Reactants are substances that start a chemical reaction, and products are substances that are produced in the reaction.
::所有化学反应都涉及活性剂和产品,活性剂是开始化学反应的物质,产品是反应中产生的物质。 -
A chemical reaction can be represented by the general chemical equation:
::一般化学方程式可以代表一种化学反应:
-
-
- Reactants → Products
-
-
Bonds break and reform during chemical reactions. Reactants and products contain the same atoms, but they are rearranged during the reaction, so reactants and products are different substances.
::在化学反应过程中,键断裂和改变。活性剂和产品含有相同的原子,但在反应期间重新排列,因此反应剂和产品是不同的物质。
Review
::回顾-
Identify the reactants and products in the following chemical reaction:
::查明下列化学反应中的反应剂和产品:
-
-
- CH 4 + 2O 2 → CO 2 + 2H 2 O
-
-
How do reactants change into products during a chemical reaction?
::在化学反应过程中,反应剂如何转化为产品?
-
Reactants
are substances that start a chemical reaction.