5.14 不可逆转和不可逆转的反应
章节大纲
-
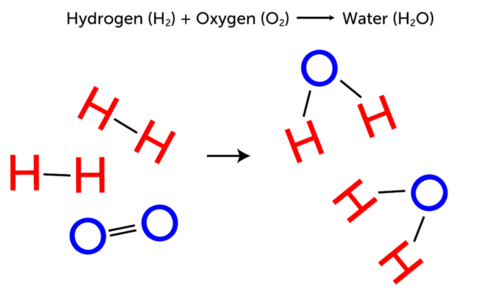
This diagram represents a . In the reaction, two molecules of hydrogen (2 H-H) combine one molecule of oxygen gas (O=O) to produce two molecules of water (2 H-O-H). Look at the arrow in the diagram. What does it represent? It shows that the reaction normally occurs in that direction, that is, from the two gases to water. Does the opposite reaction ever occur? Can water change back to hydrogen and oxygen gases? In other words, is the reaction reversible? Read on to find out.
::此图表代表 。 在反应中, 两个氢分子(2 H-H) 结合一个氧气分子( O=O) 产生两个水分子(2 H-O-H) 。 看看图表中的箭头 。 它代表什么 ? 它显示反应通常朝这个方向发生, 即从两种气体到水。 是否发生了相反的反应? 水能回溯到氢和氧气吗? 换句话说, 反应是可逆的吗? 阅读以了解 。Irreversible Reactions
::不可逆反应Some chemical reactions can occur in only one direction. These reactions are called irreversible reactions. The reactants can change to the products, but the products cannot change back to the reactants. These reactions are like making a cake. The ingredients of a cake—such as eggs and flour—are the reactants. They are mixed together and baked to form the cake, which is the product (see Figure ). The cake can’t be “unbaked” and “unmixed” to change it back to the raw eggs, flour, and other ingredients. So making a cake is irreversible.
::某些化学反应可能只有一个方向。 这些反应被称为不可逆转的反应。 反应者可以改变产品,但产品不能回到反应者身上。 这些反应就像做蛋糕一样。 蛋糕的成分 — — 如鸡蛋和面粉 — — 是反应者的成分。 它们混合在一起,烤成蛋糕,即产品(见图 ) 。 蛋糕不能“未烤”和“未混合”来将它改成生鸡蛋、面粉和其他成分。 因此蛋糕是不可逆转的。Combustion reactions are generally irreversible. Combustion occurs whenever a fuel burns. In this type of reaction, the fuel may combine with oxygen (in the air) and produces carbon dioxide and water vapor. The chemical equation for a is:
::燃烧反应一般是不可逆转的,每当燃料燃烧时会发生燃烧,在这种反应中,燃料可能与氧气(空气)结合,产生二氧化碳和水蒸气。-
-
- Fuel + O 2 → CO 2 + H 2 O
-
In a complete combustion reaction, fuel and oxygen are the reactants and the products are carbon dioxide and water. These two products cannot react to reform the fuel and oxygen, so the reaction is irreversible. The one-way arrow in the equation shows that the reaction can go in only one direction.
::在完全燃烧反应中,燃料和氧是反应剂,产品是二氧化碳和水。 这两个产品无法对燃料和氧气的改造做出反应,因此反应是不可逆转的。 方程式中的单向箭头显示反应只能朝着一个方向发展。Reversible Reactions
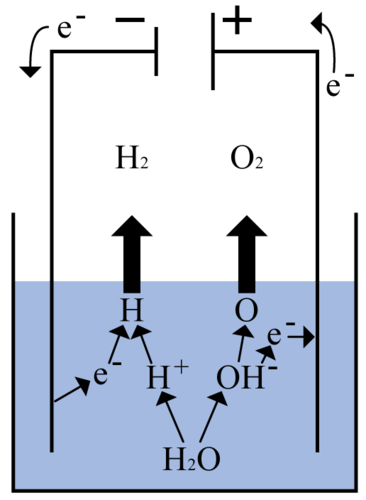
::可逆反应反应Many chemical reactions can occur in both directions. These reactions are called reversible reactions. Not only can the reactants change to the products, but the products can change back to the reactants, at least under certain conditions. Consider again the reaction in which hydrogen gas and oxygen gas combine to produce water. This reaction is reversible if an electric current is applied to the water that is produced. You can see how this is done in the Figure . The current causes the water molecules to break down to individual hydrogen and oxygen atoms. Then these atoms recombine to form molecules of hydrogen gas and oxygen gas.
::许多化学反应可能发生于两个方向。这些反应被称为可逆反应。这些反应不仅可以改变产品的反应,而且产品可以恢复到反应,至少在某些条件下是这样。再次考虑氢气和氧气结合产生水的反应。如果对产生的水使用电流,这种反应是可以逆转的。你可以看到图中是如何做到这一点的。当前水分子会分解成个别的氢和氧原子。然后这些原子会形成氢气和氧气的分子。Q: In a chemical equation, a reversible reaction is represented with two arrows, one pointing in each direction. This shows that the reaction can go both ways. How would you represent the reversible reaction in which hydrogen and oxygen gases combine to produce water?
::问题:在化学方程式中,可逆反应用两支箭表示,一箭指向每个方向。这表明反应可以双向进行。你如何代表氢气和氧气结合产生水的可逆反应?A: The chemical equation would be:
::A:化学方程式为:-
-
- H 2 + O 2 H 2 O
-
Balancing Act
::平衡法In a reversible reaction, both forward and reverse directions of the reaction generally occur at the same time. While reactants are reacting to produce products, products are reacting to produce reactants. Often, a point is reached at which forward and reverse directions of the reaction occur at the same rate. When this happens, there is no overall change in the amount of , even though the reactions keep occurring in both directions. This point is called equilibrium . The term equilibrium means “state of balance,” and it is used to refer to a state of balance between any opposing changes.
::在一种可逆反应中,反应的前向和反向方向通常同时发生。当反应者对生产产品作出反应时,产品对生产反应者作出反应时,产品对生产反应者作出反应时,往往达到一个点,即反应的前向和反向方向以同样的速度发生。当发生这种情况时,反应的数量没有总体变化,即使反应在两个方向持续发生。这个点被称为平衡。平衡一词的意思是“平衡状态 ” , 用来指任何相反的变化之间的平衡状态。Summary
::摘要-
Irreversible chemical reactions can occur in only one direction. The reactants can change to the products, but the products cannot change back to the reactants.
::不可逆转的化学反应可能只有一个方向。 反应者可以改变产品,但产品不能回到反应者身上。 -
Reversible chemical reactions can occur in both directions. The reactants can change to the products, and the products can also change back to the reactants.
::反向化学反应可能发生于两个方向。 反应器可以改变产品,产品也可以回到反应器。 -
Equilibrium occurs when forward and reverse directions of a reversible reaction occur at the same rate so there is no overall change in the amounts of reactants and products.
::当可逆反应的前向和反向方向以相同速度发生时,即出现平衡,因此反应器和产品的数量没有总体变化。
Review
::回顾-
What is an irreversible chemical reaction? Give an example.
::什么是不可逆转的化学反应?举个例子。 -
Describe a reversible chemical reaction.
::描述一种可逆化学反应。 -
When is a reversible chemical reaction in equilibrium?
::何时在平衡中出现可逆的化学反应?
-