5.24 内热反应
章节大纲
-
Did you ever use an instant ice pack like this one? You don’t have to pre-cool it in the freezer. All you need to do is squeeze the pack and it starts to get cold. How does this happen? The answer for some cold packs is an endothermic .
::你曾经使用过像这样的瞬间冰块吗? 你不必在冰箱里预先冷却冰块。 你只需要挤压冰块,它就会开始变冷。 怎么会这样呢? 一些冷冻袋的答案是最终的热量。What Is An Endothermic Reaction?
::什么是局部热反应?All chemical reactions involve . Energy is used to break bonds in reactants , and energy is released when new bonds form in products. In some chemical reactions, called , more energy is released when new bonds form in the products than is needed to break bonds in the reactants. The opposite is true of endothermic reactions. In an endothermic reaction , it takes more energy to break bonds in the reactants than is released when new bonds form in the products.
::所有化学反应都涉及。能源被用来打破反应器的联结,当产品中出现新的联结时,能源就会释放出来。在某些化学反应中,所谓的“当产品中出现新的联结时,能量就会释放出来,而当反应器中出现新的联结时,能量就会释放出来,而当反应器中出现新的联结时,能量就会释放出来,反之,内温反应则会发生。在热反应中,当反应器中出现新的联结时,能量就会释放出来,而当产品中出现新的联结时,能量就会释放出来。Energy Change in Endothermic Reactions
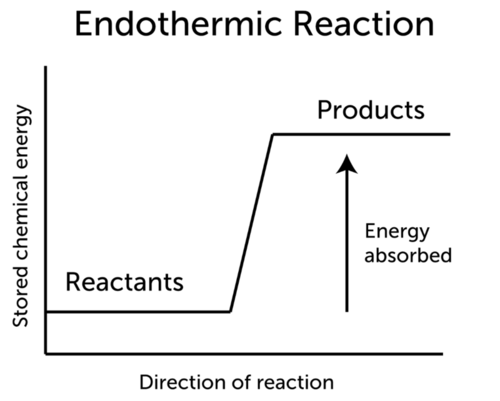
::局部热反应中的能源变化The word endothermic literally means “taking in .” A constant input of energy, often in the form of heat, is needed to keep an endothermic reaction going. This is illustrated in the Figure . Energy must be constantly added because not enough energy is released when the products form to break more bonds in the reactants. The general equation for an endothermic reaction is:
::终端热字字面意思是“吸收 ” 。 需要不断投入能量,通常以热的形式,以保持局部热反应。图中说明了这一点。 能源必须不断添加,因为当产品形成时,能量不够释放,无法打破反应器中更多的联系。 终端热反应的一般方程式是:-
-
- Reactants + Energy → Products
-
Note: ΔH represents the change in energy. In endothermic reactions, the of the products is typically lower than the temperature of the reactants. The drop in temperature may be great enough to cause to freeze.
::在内热反应中,产品通常低于反应器的温度,温度下降可能足以导致冻结。Q: Now can you guess how an instant cold pack works?
::问:现在你能猜到冷冻包是如何工作的吗?A: Squeezing the cold pack breaks an inner bag of water, and the water mixes with a chemical inside the pack. The chemical and water combine in an endothermic reaction. The energy needed for the reaction to take place comes from the water, which gets colder as the reaction proceeds.
::A:挤压冷冻袋打破了水的内袋,水与水中的化学物质混在一起。化学和水结合了局部热反应。反应所需的能量来自水,随着反应的不断推进,水会变冷。Photosynthesis
::光合作用One of the most important series of endothermic reactions is . In photosynthesis, plants make the simple sugar glucose (C 6 H 12 O 6 ) from carbon dioxide (CO 2 ) and water (H 2 O). They also release oxygen (O 2 ) in the process. The reactions of photosynthesis are summed up by this chemical equation :
::在光合作用中,植物用二氧化碳(CO2)和水(H2O)制成简单的甘蔗糖(C6H12O6),在过程中还释放氧气(O2)。光合作用的反应以化学方程式概括如下:-
-
- 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2
-
The energy for photosynthesis comes from light. Without light energy, photosynthesis cannot occur. As you can see in the Figure , plants can get the energy they need for photosynthesis from either sunlight or .
::光合作用能量来自光线。没有光能,光合作用就无法发生。正如图所示,植物可以从阳光或阳光中获取光合作用所需的能量。Summary
::摘要-
An endothermic reaction is a chemical reaction in which more energy is needed to break bonds in the reactants than is released when new bonds form in the products.
::终端热反应是一种化学反应,在化学反应中,需要比产品中新债券形成时释放更多的能量来打破反应器中的联结。 -
A constant input of energy, often in the form of heat, is needed to keep an endothermic reaction going.
::需要不断投入能量,通常以热为形式,以保持内温反应。 -
One of the most important series of endothermic reactions is photosynthesis. The energy needed for photosynthesis comes from light.
::最重要的一系列局部热反应之一是光合作用。光合作用所需的能量来自光。
Review
::回顾-
What is an endothermic reaction?
::什么是局部热反应? -
Why is the temperature of products likely to be lower than the temperature of reactants in an endothermic reaction?
::为什么产品温度可能低于内温反应反应反应反应物的温度? -
Describe an example of an endothermic reaction.
::描述一个内温反应的例子。
Resources
::资源 -