5.28催化剂催化器
章节大纲
-
The tunnel through this mountain provides a faster route for cars to get to the other side of the mountain. If a were like a road to the other side of a mountain, a catalyst would be like a tunnel.
::穿过这座山的隧道为汽车到山的另一边提供了一条更快的路线。 如果一条公路通往山的另一边,催化剂就像一条隧道。What Is A Catalyst?
::什么是催化剂?A catalyst is a substance that increases the rate of a chemical reaction. The presence of a catalyst is one of several factors that influence the rate of chemical reactions. (Other factors include the , , and surface area of reactants .) A catalyst isn’t a reactant in the chemical reaction it speeds up. As a result, it isn’t changed or used up in the reaction, so it can go on to catalyze many more reactions.
::催化剂是一种能提高化学反应速度的物质。 催化剂的存在是影响化学反应速度的若干因素之一。 (其他因素包括反应器的表面面积和反应器的面积。 )催化剂在加速的化学反应中不是反应剂。 结果,催化剂没有改变,也没有在反应中使用,因此它可以继续催化更多的反应。Q: How is a catalyst like a tunnel through a mountain?
::问:像隧道一样的催化剂如何穿过山?A: Like a tunnel through a mountain, a catalyst provides a faster pathway for a chemical reaction to occur.
::A:就像穿过山的隧道一样,催化剂为发生化学反应提供了更快的路径。How Catalysts Work
::催化器如何起作用Catalysts interact with reactants so the reaction can occur by an alternate pathway that has a lower . Activation energy is the needed to start a reaction. When activation energy is lower, more reactant particles have enough energy to react so the reaction goes faster. Many catalysts work like the one in the Figure . The catalyst brings the reactants together by temporarily bonding with them. This makes it easier and quicker for the reactants to react together.
::催化器与反应器相互作用,这样反应就可以通过另一个途径发生,而另一个途径的响应速度较低。启动反应需要激活能量。当激活能量降低时,更多的反应粒子有足够的能量来作出反应,这样反应速度就会更快。许多催化剂像图中的催化剂那样工作。催化剂通过临时结合将反应器聚集在一起。这使得反应器更容易和更快地一起反应。Q: In the Figure , look at the energy needed in the catalytic and non-catalytic pathways of the reaction. How does the amount of energy compare? How does this affect the along each pathway?
::问题:在图中,看看反应的催化和非催化路径所需的能量。能量量如何比较?这如何影响每个路径?A: The catalytic pathway of the reaction requires far less energy. Therefore, the reaction will occur faster by this pathway because more reactants will have enough energy to react.
::A:反应的催化途径需要的能量要少得多。 因此,反应会更快地通过这条途径发生,因为更多的反应者将有足够的反应能量。Catalysts in Living Things
::生命中的催化剂Chemical reactions constantly occur inside living things. Many of these reactions require catalysts so they will occur quickly enough to support life. Catalysts in living things are called . Enzymes may be extremely effective. A reaction that takes a split second to occur with an enzyme might take many years without it!
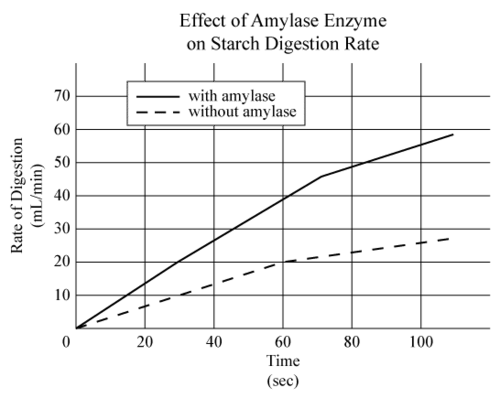
::化学反应经常发生在生物中。许多这些反应需要催化剂,这样它们就能迅速产生,从而支持生命。生命中的催化剂被称为 。酶可能非常有效。用酶进行反应需要很多年的时间。More than 1000 different enzymes are necessary for human life. Many enzymes are needed for the digestion of food. An example is amylase, which is found in the mouth and small intestine. Amylase catalyzes the breakdown of starch to sugar. You can see how it affects the rate of starch digestion in the Figure .
::人类生命需要1000多种不同的酶。食物消化需要许多酶。例如,在口腔和小肠中发现的氨基酶。Amylase将淀粉分解成糖。你可以看到它如何影响图中的淀粉消化速度。Q: If you chew a starchy food such as a soda cracker for a couple of minutes, you may notice that it starts to taste slightly sweet. Why does this happen?
::问题:如果你在几分钟内咀嚼苏打饼干等星空食物,你可能会注意到它开始有点甜。为什么会这样呢?A: The starches in the cracker start to break down to sugars with the help of the enzyme amylase. Try this yourself and see if you can taste the reaction.
::A: 饼干中的淀粉在酶氨基酶的帮助下开始分解成糖。 自己试试这个, 看看能不能尝到反应的味道 。Summary
::摘要-
A catalyst is a substance that increases the rate of a chemical reaction.
::催化剂是一种可提高化学反应率的物质。 -
A catalyst provides an alternate pathway for the reaction that has a lower activation energy. When activation energy is lower, more reactant particles have enough energy to react, so the reaction occurs faster.
::催化剂为激活能量较低的反应提供了替代路径。 当激活能量较低时, 更多的反应粒子有足够的反应能量, 因此反应会更快发生。 -
Chemical reactions constantly occur inside living things, and many of them require catalysts to occur quickly enough to support life. Catalysts in living things are called enzymes.
::化学反应经常发生在生物中,其中许多需要催化剂快速地产生,以维持生命。 生命中的催化剂被称为酶。
Review
::回顾-
What is a catalyst?
::什么是催化剂? -
How does a catalyst speed up a chemical reaction?
::催化剂如何加速化学反应? -
What are enzymes? Why are they important?
::什么是酶 为什么它们重要
Explore More
::探索更多Watch the video showing a chemical reaction both with and without a catalyst. Then answer the questions below.
::视频显示一种化学反应 与催化剂和没有催化剂。然后回答下面的问题。-
Write the chemical equation for the reaction that is demonstrated in the video.
::为视频中显示的反应写化学方程式。 -
What chemical is used to catalyze the reaction?
::用来催化反应的化学物质是什么? -
Describe two observations that provide evidence that the reaction has occurred after the addition of the catalyst.
::说明两点意见,以提供证据,证明该反应是在添加催化剂之后发生的。
-
A catalyst is a substance that increases the rate of a chemical reaction.