6.6 Isomer
章节大纲
-
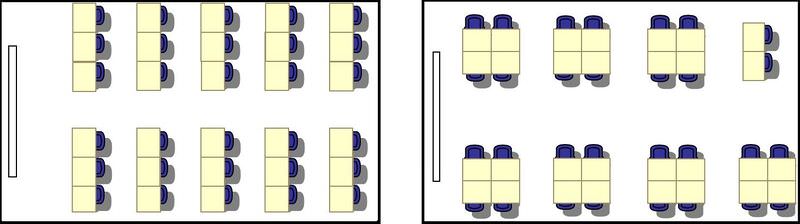
Look at the desks in the images above. Both pictures show the same classroom with the same number of desks, but the desks are arranged differently in each picture. The different arrangements work better for different classroom activities. Desks in rows facing the front of the classroom are better for watching the teacher do demonstrations. Desks facing each other in small groups are better for working on group projects. What does the arrangement of desks in a classroom have to do with chemistry ? Like desks in a classroom, atoms in a molecule can be arranged in different ways, and the different arrangements give them different properties.
::以上图像中的课桌。 这两张图片显示的是相同的教室, 同样的课桌数量相同, 但每张课桌的排列不同。 不同的教室活动安排不同。 不同的教室活动安排更好。 站在教室前排的课桌更适合看教师进行演示。 以小组小组形式面对面的课桌更适合小组项目工作。 教室的课桌安排与化学有什么关系? 像教室的课桌一样, 分子中的原子可以以不同的方式安排, 不同的安排可以给予他们不同的属性 。Same Atoms, Different Shapes
::相同原子, 不同形状Hydrocarbons are compounds that contain only carbon and hydrogen atoms. The smallest hydrocarbon, methane (CH 4 ), contains just one carbon and four hydrogen atoms. Larger hydrocarbons contain many more. Hydrocarbons with four or more carbon atoms can have different shapes. Although they have the same , with the same numbers of carbon and hydrogen atoms, they form different compounds, called . Isomers are compounds whose properties are different because their atoms are bonded together in different arrangements.
::碳氢化合物是仅含有碳和氢原子的化合物。最小的碳氢化合物,甲烷(CH4)仅含有一个碳和四个氢原子。更大的碳氢化合物含有更多的碳。拥有四个或四个以上碳原子的碳氢化合物可以有不同的形状。尽管碳和氢原子的数量相同,但它们构成不同的化合物,被称为。因原子在不同的安排中结合在一起而性质不同的化合物。Examples of Isomers
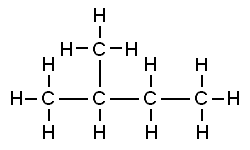
::Isomerers 实例The smallest hydrocarbon that has isomers is butane, which has just four carbon atoms. In the Figure you can see structural formulas for normal butane (or n -butane) and its only isomer, named iso -butane. Both molecules have four carbon atoms as well as ten hydrogen atoms (C 4 H 10 ), but the atoms are arranged differently in the two compounds. In n -butane, all four carbon atoms are lined up in a straight chain. In iso -butane, one of the carbon atoms branches off from the main chain.
::有异构体的最小碳氢化合物是丁烷,它只有四个碳原子。在图中,您可以看到普通丁烷(或非丁烷)及其唯一异构体(称为异丁烷)的结构公式。两种分子都有四个碳原子和十个氢原子(C4H10),但在两种化合物中原子的排列方式不同。在正丁烷中,所有四个碳原子都排在一条直线链中。在异丁烷中,一个碳原子从主链中分离出来。The next smallest hydrocarbon is pentane, which has five carbon atoms and twelve hydrogen atoms (C 5 H 12 ). Pentane has three isomers: n -pentane, iso -pentane, and neo -pentane. Their structural formulas are shown in the images below. Look at the carbon atoms in each isomer. In n -pentane (see Figure ), the carbon atoms form a straight chain. In iso -pentane (see Figure ), one carbon atom branches off from the main chain. In neo -pentane (see Figure ), two carbon atoms branch off from the main chain.
::下一个最小的碳氢化合物是五溴二苯醚,它有5个碳原子和12个氢原子(C5H12)。 Pentane有3个异构体: n-pentane, iso-pentane, eto-pentane, 和 n-pentane, 和 n-pentane, 其结构公式见于下图中。 查看每个异构体的碳原子。 在 n- pentane (见图 ) 中, 碳原子形成直线链。 在Iso- pentane(见图 ) 中, 一个碳原子分支从主链中分离出来。 在新- pentane(见图 ) 中, 两个碳原子分支从主链中分离出来。How Many Isomers?
::有多少异教徒?Butane has only two isomers and pentane has just three, but some hydrocarbons have many more isomers than these. As you increase the number of carbon atoms in a hydrocarbon, the number of isomers quickly increases. For example, heptane, with seven carbon atoms, has nine isomers; and dodecane, with twelve carbon atoms, has 355 isomers. Some hydrocarbons with many more carbon atoms have billions of isomers!
::丁烷只有两个异构体, 五溴二苯醚只有三个, 但有些碳氢化合物有比这三个异构体更多的异构体。 当你增加碳原子在碳氢化合物中的数量时, 异构体的数量会迅速增加。 比如, 七烷有七个碳原子, 有九个异构体; 多烷有十二个碳原子, 有355个异构体。 有些碳原子更多的碳氢化合物有数十亿个异构体!Q: Why does the number of carbon atoms in a hydrocarbon determine how many isomers it has?
::问题:为什么碳原子在碳氢化合物中的数量决定其有多少异构体?A: The more carbon atoms there are, the greater the number of possible arrangements of carbon atoms.
::A:碳原子越多,碳原子的可能安排越多。Properties of Isomers
::异异物属性Because isomers are different compounds, they have different properties. Generally, branched-chain isomers have lower and points than straight-chain isomers. For example, the boiling and melting points of iso -butane are -12 °C and -160 °C, respectively, compared with 0 °C and -138 °C for n -butane. The more branching there is, the lower the boiling and melting points are.
::由于异构体是不同的化合物,因此它们具有不同的特性。一般而言,分链异构体比直链异构体低和中点。例如,异丁烷的沸点和熔点分别为 -12 °C 和 -160 °C,而正丁烷的沸点和熔点分别为 0 °C 和 - 138 °C。分环越多,沸点和熔点就越低。Q: The boiling point of n -pentane is 36 °C. Predict the boiling points of iso -pentane and neo -pentane.
::Q: 正丙烷的沸点为36°C。预测异丙烷和新丙烷的沸点。A: The boiling point of iso -pentane is 28 °C, and the boiling point of neo -pentane is 10 °C.
::A:异丙烷的沸点为28°C,新丙烷的沸点为10°C。Summary
::摘要-
Hydrocarbons with the same numbers of atoms but different shapes form different compounds called isomers.
::原子数量相同但形状不同的碳氢化合物构成不同的化合物,称为异构体。 -
Hydrocarbons with four or more carbon atoms have isomers. The more carbon atoms a hydrocarbon has, the greater the number of isomers.
::有四个或四个以上碳原子的碳氢化合物有异构体,碳氢化合物的碳原子越多,异构体的数量就越多。 -
Isomers are different compounds with different properties, such as different boiling and melting points.
::异构体是具有不同特性的不同化合物,例如不同的沸点和熔点。
Review
::回顾-
What are isomers?
::什么是异构体? -
Name and describe the isomers of butane.
::名称和描述丁烷异构体。 -
Identify the hydrocarbon that has nine isomers. What is its chemical formula?
::识别含有九种异构体的碳氢化合物。其化学公式是什么?
Explore More
::探索更多Watch the video about isomers of hexane. Then answer the questions below.
::观看关于六溴二苯醚异构体的视频。然后回答下面的问题。-
What is the chemical formula for hexane?
::何为六氯丁二烯的化学公式? -
How many isomers does hexane form?
::有多少异构体是六氯代苯形成的? -
How do boiling points of hexane isomers vary?
::何以六溴异构体的沸点不同?
Resources
::资源 -
Hydrocarbons with the same numbers of atoms but different shapes form different compounds called isomers.