8.5 阿尔法衰变
章节大纲
-
You probably associate the term decay with images like the one above. But when it comes to atoms, decay has a different meaning. Decay in chemistry refers to changes in the nuclei of certain atoms.
::您可能将“ 衰变” 一词与上述图像联系起来。 但对于原子来说, 衰变有不同的含义。 化学衰变是指某些原子核心的变化 。Why Some Nuclei Decay
::为什么有些纽克莱德凯Radioactive and isotopes have unstable nuclei. To become more stable, the nuclei undergo . In radioactive decay, the nuclei give off, or emit, radiation in the form of and often particles as well. There are several , including alpha, beta, and . Energy is emitted in all three types of decay, but only alpha and also emit particles.
::放射性和同位素有不稳定的核。要变得更稳定,核将经历。在放射性衰变中,核也以粒子的形式释放或释放辐射。有几种辐射,包括α、β和。能量在所有三种类型的衰变中都释放出来,但只有α和释放颗粒。What Is Alpha Decay?
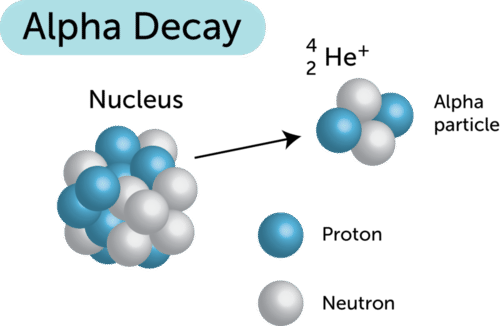
::什么是阿尔法衰变?Alpha decay occurs when a nucleus is unstable because it has too many . The Figure shows what happens during alpha decay. The nucleus emits an alpha particle and energy. An alpha particle consists of two protons and two , which is actually a helium nucleus. Losing the protons and neutrons makes the nucleus more stable.
::Alpha 衰变发生在核不稳定时,因为它有太多。图中显示了在α衰变期间发生的情况。核心释放了α粒子和能量。一个阿尔法粒子由两个质子和两个粒子组成,这实际上是氦核。失去质子和中子使核更加稳定。Equations for Alpha Decay
::阿尔法衰变的方形Radioactive nuclei and particles are represented by nuclear symbols that indicate their numbers of protons and neutrons. For example, an alpha particle (helium nucleus) is represented by the symbol , where He is the chemical symbol for helium, the subscript 2 is the number of protons, and the superscript 4 is the mass number (2 protons + 2 neutrons).
::放射性核和粒子由核符号代表,核符号表示其质子和中子的数量。例如,一个α粒子(核)以符号24H(He)表示,他就是的化学符号,下标2是质子的数量,上标4是质数(2质子+2中子)。Nuclear symbols are used to write nuclear equations for radioactive decay. Let’s consider an example. Uranium-238 undergoes alpha decay to become thorium-234. (The numbers following the chemical names refer to the number of protons plus neutrons.) In this reaction, uranium-238 loses two protons and two neutrons to become the element thorium-234. The reaction can be represented by this nuclear equation:
::核符号被用来为放射性衰变写核方程式。让我们举一个例子。铀238会发生α衰变,变成234。 (化学名称后面的数字指质子加中子的数量。 )在这种反应中,铀238会损失两个质子和两个中子,成为元素234。 反应可以用这个核方程式来表示:-
-
- → + + Energy
-
If you count the number of protons (subscripts) as well as the number of protons plus neutrons (superscripts), you’ll see that the total numbers are the same on both sides of the arrow. This means that the equation is balanced. The thorium-234 produced in this reaction is also unstable, so it will undergo radioactive decay as well. The alpha particle ( ) produced in the reaction can join with two free electrons to form the element helium. This is how most of Earth’s helium formed.
::如果您计算质子的数量( 下标) 和质子加中子的数量( 上标) , 你就会看到箭头两侧的总数都是一样的。 这意味着方程式是平衡的。 以这种反应产生的-234也不稳定, 因而也会发生放射性衰变。 反应中产生的α粒子( 24He) 与两个自由电子结合形成元素。 这就是地球大部分的形成的方式。Q: Fill in the missing subscript and superscript to balance the following nuclear equation for alpha decay of Polonium-210.
::问题:填充缺失的下标和上标,以平衡下列-210的α衰变的核方程式。-
-
- → + + Energy
-
A: The subscript of Pb is 82, and the superscript is 206. This means that the new element produced in the reaction has 82 protons. You can find the element with this number of protons in the periodic table . It is the element lead (Pb). The new element also has 124 neutrons (206 – 82 protons = 124 neutrons).
::A: Pb 的下标为 82, 上标为 206。 这意味着在反应中产生的新元素有 82 质子。 您可以在周期表中找到此元素, 质子数量在周期表中。 这是元素铅 (Pb) 。 新元素还有 124 个中子( 206 - 82 质子= 124 中子 ) 。How Dangerous Is Alpha Decay?
::阿尔法衰变有多危险?All types of radioactive decay pose risks to living things, but alpha decay is the least dangerous. That’s because alpha particles are relatively heavy, so they can travel only a few centimeters through the air. They also are not very penetrating. For example, they can’t pass through a sheet of paper or thin layer of clothing. They may burn the skin, but they can’t penetrate to the tissues underneath the skin. However, if alpha particles are emitted inside the body, they can do more damage. One way this can happen is by inhaling cigarette smoke. People who smoke actually inhale the radioactive element polonium-210. It undergoes alpha decay in the lungs. Over time, exposure to alpha particles may cause lung cancer.
::所有类型的放射性衰变都对生物构成危险,但α衰变是最危险的东西。这是因为α粒子相对较重,因此它们只能在空气中穿梭几厘米。它们也不太渗透。例如,它们不能穿透纸片或薄层的衣物。它们可能烧伤皮肤,但不能渗透到皮肤下的组织中。然而,如果α粒子在体内释放,它们可以造成更多的损害。其中一个办法是吸入烟雾。吸烟者实际上吸入放射性元素210。它会经历肺部的甲状腺腐蚀。随着时间的推移,接触甲状腺粒子可能会导致肺癌。Summary
::摘要-
Alpha decay is one of three types of nuclear decay in which unstable nuclei emit energy with or without a particle of matter.
::阿尔法的衰变是三种核衰变类型之一,其中不稳定的核核循环会释放能量,无论有无物质粒子。 -
In alpha decay, energy and an alpha particle are emitted by a nucleus that is unstable because it has too many protons. An alpha particle consists of two protons and two neutrons, so it is actually a helium nucleus.
::在阿尔法衰变中,能量和阿尔法粒子是由一个因质子过多而不稳定的核释放出来的。一个阿尔法粒子由两个质子和两个中子组成,因此实际上它是一个氦核。 -
Alpha decay is represented by a nuclear equation. The equation is balanced if the total numbers of protons and neutrons are the same on both sides of the arrow.
::Alpha 衰变由核方程式表示。如果箭头两侧质子和中子的总数相同,则方程式是平衡的。 -
All radioactive decay is dangerous to living things, but alpha decay is the least dangerous.
::所有放射性衰变都对生物有危险 但阿尔法衰变最危险
Review
::回顾-
What is alpha decay?
::什么是阿尔法衰变? -
Explain why alpha decay occurs.
::解释为什么阿尔法衰变会发生 -
If a radioactive element with 85 protons undergoes alpha decay, how many protons will there be in the new element that forms as the product of the reaction? What element is it?
::如果含有85个质子的放射性元素发生阿尔法衰变,作为反应产物的新元素中将有多少质子?什么元素? -
Fill in the missing subscript and superscript to balance the following nuclear equation. Make sure your equation is balanced.
::填入缺失的下标和上标以平衡以下核方程式。 确保您的方程式平衡 。
- → + + Energy
Resources
::资源 -