8.9 辐射日期
章节大纲
-
The Grand Canyon, pictured above, was carved by the rushing waters of the Colorado River over millions of years. The exposed rocks at the bottom of the canyon are almost 2 billion years old. The youngest rocks near the top are about 230 million years old. Therefore, from top to bottom, the rocks provide a continuous record of more than 1.5 billion years of geological history in this region.
::上图所示的大峡谷由科罗拉多河喷涌的水域在数以百万年的时间里雕刻。峡谷底部暴露的岩石有近20亿年的历史,顶部附近的最年轻的岩石有约2.3亿年的历史。 因此,从上到下,岩石提供了该地区15亿年地质史的连续记录。Q: How have scientists been able to determine the ages of rocks in the Grand Canyon?
::问:科学家如何确定大峡谷岩石的年代?A: The ages are based on the gradual decay, or break down, of radioactive isotopes.
::A: 年龄基于放射性同位素的逐渐衰减或分解。What Is Radioactive Dating?
::辐射约会是什么?Radioactive isotopes, or , can be used to estimate the ages of not only of rocks, but also of fossils and artifacts made long ago by human beings. Even the age of Earth has been estimated on the basis of radioisotopes. The general method is called radioactive dating . To understand how radioactive dating works, you need to understand radioisotopes and .
::放射性同位素或放射性同位素可以用来估计不仅岩石的年代,而且还可以估计人类很久以前制造的化石和文物的年代。甚至地球的年代也是根据放射性同位素估计的。一般方法称为放射性约会。为了了解放射性约会如何起作用,你需要了解放射性同位素和放射性同位素。Radioisotopes and Radioactive Decay
::放射性同位素和放射性衰减A radioisotope has atoms with unstable nuclei . Unstable nuclei naturally decay, or break down. They lose and particles and become more stable. As nuclei decay, they gain or lose , so the atoms become different . This is illustrated in the Figure . The original, unstable nucleus is called the parent nucleus. After it loses a particle (in this case a type of particle called an alpha particle), it forms a daughter nucleus, with a different number of protons.
::放射性同位素有不稳定核的原子。 不稳定的核自然衰变或分解。 它们会丢失和颗粒, 并变得更加稳定。 随着核衰变, 它们会增减, 原子会变得不同 。 图中说明了这一点。 原始的不稳定核被称为母核。 当它失去一个粒子( 一种叫做甲型粒子的粒子) 之后, 它会形成一个子核, 并有不同的质子数量 。The nucleus of a given radioisotope decays at a constant rate that is unaffected by , pressure , or other conditions outside the nucleus. This rate of decay is called the . The half-life is the length of time it takes for half of the original amount of the radioisotope to decay to another element.
::特定放射性同位素核心以恒定速度衰减,不受核外压力或其他条件的影响。这种衰变速度被称为 。半衰期是放射性同位素原量的一半衰减到另一个元素所需的时间长度。Q: How can the half-life of a radioisotope be used to date a rock?
::问题:如何将放射性同位素的半衰期用于与岩石约会?A: After a rock forms, nuclei of a radioisotope inside the rock start to decay. As they decay, the amount of the original, or parent, decreases, while the amount of its stable decay product, or daughter isotope, increases. By measuring the relative amounts of parent and daughter isotopes and knowing the rate of decay, scientists can determine how long the parent isotope has been decaying. This provides an estimate of the rock’s age.
::A:在岩石形式之后,岩石中的放射性同位素核开始衰减。随着原同位素或母同位素的衰减,原同位素或母同位素的数量会减少,而其稳定的衰变产物或女同位素的数量会增加。 通过测量母同位素和女同位素的相对数量,并了解衰变速度,科学家可以确定母同位素的衰减时间。 这提供了对岩石年代的估计。Different Isotopes, Different Half-Lives
::不同的异形、不同的半生、不同的半生Different radioisotopes decay at different rates. You can see some examples in the Table . Radioisotopes with longer half-lives are used to date older rocks or other specimens, and those with shorter half-lives are used to date younger ones. For example, the oldest rocks at the bottom of the Grand Canyon were dated by measuring the amounts of potassium-40 in the rocks. Carbon-14 dating, in contrast, is used to date specimens that are much younger than the rocks in the Grand Canyon. You can read more carbon-14 dating below.
::不同的放射性同位素以不同的速度衰减。 您可以在表格中看到一些例子。 使用半衰期较长的放射性同位素来与老的岩石或其他标本进行约会, 使用半衰期较短的标本来与较年轻的标本进行约会。 例如, 大峡谷底最古老的岩石通过测量岩石中的钾-40含量而过时。 相比之下, 碳-14 日期被用来与比大峡谷岩石更小的标本进行约会。 您可以在下面阅读更多的碳-14 日期 。Parent Isotope Daughter Isotope Half-Life potassium-40 argon-40 1.3 billion years uranium-235 lead-207 700 million years uranium-234 thorium-230 80,000 years carbon-14 nitrogen-14 5,700 years Focus on Carbon-14 Dating
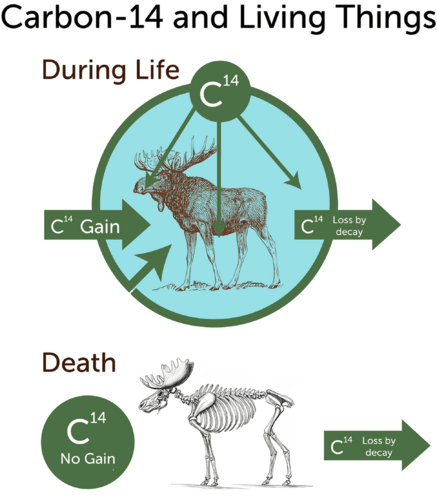
::关注碳-14日期One of the most familiar types of radioactive dating is carbon-14 dating. Carbon-14 forms naturally in Earth’s atmosphere when cosmic rays strike atoms of nitrogen-14. Living things take in and use carbon-14, just as they do carbon-12. The carbon-14 in living things gradually decays to nitrogen-14. However, as it decays, it is constantly replaced because living things keep taking in carbon-14. As a result, there is a constant ratio of carbon-14 to carbon-12 in organisms as long as they are alive. This is illustrated in the top part of the Figure .
::最熟悉的放射性约会类型之一是碳14约会。当宇宙射线击中氮14原子时,碳14自然在地球大气中形成。生命体吸收并使用碳14,就像它们使用碳12一样。生物体中的碳14逐渐衰减为氮14。然而,随着其衰变,它不断被替换,因为生物体不断吸收碳14。结果,生物体中碳14与碳12在生物体中持续的比例是恒定的,只要它们还活着。图的顶部部分说明了这一点。After organisms die, the carbon-14 they already contain continues to decay, but it is no longer replaced (see the bottom part of the Figure ). Therefore, the carbon-14 in a dead organism constantly declines at a fixed rate equal to the half-life of carbon-14. Half of the remaining carbon-14 decays every 5,700 years. If you measure how much carbon-14 is left in a fossil, you can determine how many half-lives (and how many years) have passed since the organism died.
::生物死亡后,它们已经含有的碳-14在继续衰减,但不再被替换(见图底部分 ) 。 因此, 死亡生物体中的碳-14在固定速率上持续下降, 等于碳-14的半衰期。 剩下的碳-14的一半每5700年衰减一半。 如果您测量化石中有多少碳-14还留在化石中, 您可以确定自该生物死亡以来的半衰期( 和多少年) 。Q: Why can’t carbon-14 dating be used to date specimens older than about 60,000 years?
::问:为什么不能用碳-14约会来约会年龄超过60,000年的样本?A: Carbon-14 has a half-life of 5700 years. After about 60,000 years, too little carbon-14 is left in a specimen to be measured.
::A:碳-14的半衰期为5700年。在大约60,000年之后,碳-14被留在一个样本中,无法测量。Summary
::摘要-
The age of a rock or other specimen can be estimated from the remaining amount of a radioisotope it contains and the radioisotope’s known rate of decay, or half-life. This method of dating specimens is called radioactive dating.
::岩石或其他标本的年龄可以根据其所含放射性同位素的剩余数量以及放射性同位素已知衰变速度或半衰期来估计。 这种与标本约会的方法被称为放射性约会。 -
Radioisotopes with longer half-lives are used to date older specimens, and those with shorter half-lives are used to date younger ones.
::使用半衰期较长的放射性同位素与较老的标本进行约会,使用半衰期较短的标本与较年轻的标本进行约会。 -
Carbon-14 dating is used to date specimens younger than about 60,000 years old. It is commonly used to date fossils of living things and human artifacts.
::碳-14日期用于约6万年前的标本,通常用于生物和人类文物的化石。
Review
::回顾-
What is radioactive dating?
::什么是放射性约会? -
Which radioisotope in the
Table
could you use to date a fossil thought to be about 500 million years old? Explain your choice.
::表格中哪一种放射性同位素能用来约会一个被认为有5亿年历史的化石?解释一下你的选择。 -
Why does the amount of carbon-14 in an organism remain the same throughout the organism’s life? Why does the amount change after the organism dies?
::为什么一个生物体中的碳-14数量在整个生物体生命中保持不变? 为什么生物体死亡后数量会发生变化?
Resources
::资源 -
The age of a rock or other specimen can be estimated from the remaining amount of a radioisotope it contains and the radioisotope’s known rate of decay, or half-life. This method of dating specimens is called radioactive dating.