4.10 中子
章节大纲
-
Was Sherlock Holmes real or the product of someone's imagination?
::夏洛克·福尔摩斯是真实的 还是某人想象力的产物?The most famous detective in history and literature never existed. Sherlock Holmes was the creation of the British author Sir Arthur Conan Doyle. This mythical person had capabilities far beyond those of mere mortals. Holmes was capable of spotting the tiniest clue, the smallest piece of evidence to solve the crime. He could link all sorts of seemingly irrelevant data into a coherent whole to clear up whatever mystery he was dealing with.
::历史和文学史上最著名的侦探从未存在过。 夏洛克·福尔摩斯是英国作家阿瑟·柯南·多尔爵士的创建者。 这个神话人物的能力远远超出凡人的能力。 福尔摩斯能够找到最微小的线索 — — 最小的证据 — — 来破案。 他可以将各种看似无关的数据与一个连贯的整体联系起来,以澄清他所处理的谜团。The Quest for the Neutron
::中子的追寻Clues are generally considered to involve the presence of something – a footprint, a piece of fabric, a bloodstain, something tangible that we can measure directly. The discoveries of the and the were accomplished with the help of those kinds of clues. experiments showed both the negatively charged electrons emitted by the cathode and the positively charged proton (also emitted by the cathode). The neutron was initially found not by a direct , but by noting what was not found.
::通常认为阴极和正电荷质子(也由阴极释放 ) 所释放的负电荷电子都由阴极和正电荷质子(也由阴极释放 ) 表现出来。 中子最初不是由直线发现的,而是通过指出未发现的东西而发现的。had shown the properties of the electron and the proton. Scientists learned that approximately 1,837 electrons weighed the same as one proton. There was evidence to suggest that electrons went around the heavy nucleus composed of protons. Charge was balanced with equal numbers of electrons and protons which made up an electrically neutral . But there was a problem with this model – the (number of protons) did not match the atomic weight . In fact, the atomic number was usually about half the atomic weight. This indicated that something else must be present. That something must weigh about the same as a proton, but could not have a charge – this new particle had to be electrically neutral.
::科学家们了解到,大约1,837个电子体重与一个质子重重。 有证据表明,电子环绕质子组成的重核。 电荷与构成电子中和的等量电子和质子是平衡的。 但这一模型存在问题 — — (质子数量)与原子重量不符。 事实上,原子数量通常约为原子重量的一半左右。 这表明必须存在其他东西。 某些东西必须像质子一样重,但不能有电荷 — — 这一新粒子必须是电中和的。In 1920, Ernest Rutherford tried to explain this phenomenon through the presence of another particle in the nucleus. He proposed that the "extra" particles were protons and electrons that had combined into a new particle in the nucleus (this did not turn out to be the case). These new particles would have a mass very similar to a proton, but would be electrically neutral since the positive charge of the proton and the negative charge of the electron would cancel each other out.
::1920年,欧内斯特·卢瑟福试图通过核中存在另一个粒子来解释这一现象。 他提议“超量”粒子是质子和电子,它们已经合并成核中的新粒子(情况并非如此 ) 。 这些新粒子的质子质量与质子非常相似,但会保持电子中性,因为质子的正电荷和电子的负电荷会相互抵消。In 1930, German researchers bombarded the beryllium with alpha particles (helium nuclei containing two protons and two neutrons with a charge of +2). The particles produced in this process had strong penetrating power, which suggested they were fairly large. In addition, they were not affected by a magnetic field, so they were electrically neutral. The French husband-wife research team of Frederic and Irene Joliot-Curie used these new "rays" to bombard paraffin, which was rich in protons. The unknown particles produced a large emission of protons from the paraffin.
::1930年,德国研究人员用α粒子(核,含有2个质子和2个中子,配有+2)轰炸了,在这一过程中产生的颗粒具有很强的穿透力,表明它们相当大。此外,它们没有受到磁场的影响,因此它们是中电的。Frederic和Irene Joliot-Curie的法国丈夫-妻子研究小组用这些新的“射线”来轰炸富含质子的石蜡。未知粒子从石蜡中大量排放质子。The English physicist James Chadwick (1891-1974) repeated these experiments and studied the energy of these particles. By measuring velocities, he was able to show that the new particle has essentially the same mass as a proton. So we now have a third subatomic particle with a mass equal to that of a proton, but with no charge. This particle is called the neutron. Chadwick won the Nobel Prize in Physics in 1935 for his research.
::英国物理学家詹姆斯·查德维克(James Chadwick)(1891-1974)重复了这些实验,并研究了这些粒子的能量。通过测量速度,他能够证明新粒子与质子的质量基本相同。因此我们现在有了第三个亚原子粒子,其质量相当于质子,但没有电荷。这个粒子被称为中子。查德维克在1935年获得了诺贝尔物理奖,用于他的研究。Neutron Applications
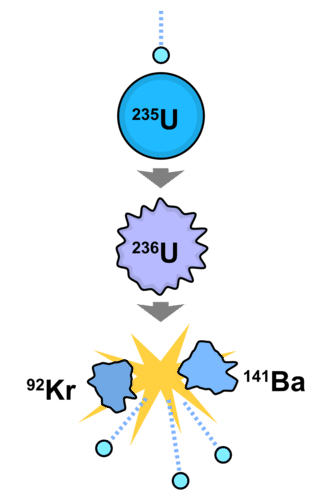
::中子应用Neutrons can be used in a variety of ways. One important use is in nuclear fission to produce new isotopes. A neutron will collide with a large atom (such as uranium) and cause it to split into smaller atoms, such as in Figure .
::中子可以多种方式使用。其中一个重要的用途是核裂变产生新同位素。中子会与大型原子(如铀)相撞,并导致其分裂成较小的原子,如图中所示。A neutron collides with a large atom, splitting it into smaller atoms.
::中子与大原子相撞, 将其分成小原子。Nuclear reactors utilize chain reactions involving neutrons to water which drive turbines for the generation of electricity. When a neutron collides with a large atom, the atom splits with the release of more neutrons and also a large amount of energy. The energy converts water to steam for the turbine, while the neutrons serve to continue the chain reaction (see Figure ).
::核反应堆利用中子对水的连锁反应,驱动涡轮机发电,当中子与大原子相撞时,原子与释放更多的中子和大量能量分解,能源将涡轮的水转化为蒸汽,而中子则继续连锁反应(见图)。How nuclear fission produces new isotopes.
::核裂变如何产生新的同位素。Summary
::摘要-
Rutherford proposed that "extra" particles in nucleus were combinations of protons and electrons.
::卢瑟福提议核中的“外”粒子是质子和电子的组合。 -
Bombardment of beryllium with alpha particles produced large, neutral particles.
::用α粒子轰炸会产生大而中性的粒子。 -
Chadwick determined the mass of the neutron.
::查德威克决定了中子的质量 -
Nuclear fission produces new elements.
::核裂变产生新的元素。 -
Nuclear reactors use chain reactions to produce heat.
::核反应堆利用连锁反应产生热量。
Review
::回顾-
How did Rutherford try to explain the differences between the number of protons in the nucleus and the atomic weight?
::卢瑟福如何解释核质子数量与原子重量之间的差别? -
What did German researchers find when they bombarded beryllium with alpha particles?
::当德国研究人员用α粒子轰炸时 发现了什么? -
What did Chadwick determine about these new particle (observed by the German scientist and the Curies)?
::Chadwick对这些新粒子(德国科学家和库里人观察的)决定了什么呢?
-
Rutherford proposed that "extra" particles in nucleus were combinations of protons and electrons.